

植物生态学报 ›› 2021, Vol. 45 ›› Issue (3): 286-297.DOI: 10.17521/cjpe.2020.0241
收稿日期:2020-07-17
接受日期:2020-10-22
出版日期:2021-03-20
发布日期:2021-05-17
通讯作者:
李强
作者简介:* E-mail: liqiang@iga.ac.cn基金资助:
LI Qiang( ), HUANG Ying-Xin, ZHOU Dao-Wei, CONG Shan
), HUANG Ying-Xin, ZHOU Dao-Wei, CONG Shan
Received:2020-07-17
Accepted:2020-10-22
Online:2021-03-20
Published:2021-05-17
Contact:
LI Qiang
Supported by:摘要:
豆科草本植物固氮是陆地生态系统重要的自然氮输入方式, 影响着草地生产的经济性和可持续性。为探讨氮磷交互作用影响豆科草本植物生物固氮率的潜在生理生态机制, 该研究选取8种豆科草本植物分别种植在对照、氮肥添加、磷肥添加和氮磷耦合添加处理的土壤中, 进行野外盆栽实验。测定了初花期植物生物量和营养含量、根部碳水化合物含量、根际pH、根际柠檬酸含量、根际有效磷含量、植物根瘤生物量、磷含量及其生物固氮率。主要结果: 依赖于豆科物种, 氮添加显著促进了豆科草本植物根际磷的活化, 降低了根生物量分配以及根系非结构性碳水化合物含量。在两种磷添加处理下, 氮添加导致8种豆科草本植物根瘤生物量平均下降27%-36%, 生物固氮率平均下降20%-33%。磷添加降低了根际的磷活化, 但促进了豆科草本植物根系发育和非结构性碳水化合物的积累。在施氮和不施氮条件下, 磷添加分别使8种豆科草本植物的生物固氮率提高了45%-69%和0-47%。氮添加降低豆科草本植物生物固氮率, 其原因是氮添加提高了植物磷需求, 为活化更多磷, 豆科草本植物降低根系生物量和根系非结构性碳水化合物的含量, 导致根瘤发育受到限制。在氮添加的同时进行磷添加, 能够改善土壤氮磷平衡, 促进根系生长和非结构性碳水化合物积累, 缓解了增氮对生物固氮的抑制作用。
李强, 黄迎新, 周道玮, 丛山. 土壤氮磷添加下豆科草本植物生物固氮与磷获取策略的权衡机制. 植物生态学报, 2021, 45(3): 286-297. DOI: 10.17521/cjpe.2020.0241
LI Qiang, HUANG Ying-Xin, ZHOU Dao-Wei, CONG Shan. Mechanism of the trade-off between biological nitrogen fixation and phosphorus acquisition strategies of herbaceous legumes under nitrogen and phosphorus addition. Chinese Journal of Plant Ecology, 2021, 45(3): 286-297. DOI: 10.17521/cjpe.2020.0241
| 处理 Treatment | 有效氮 Available N (mg·kg-1) | 有效磷 Available P (mg·kg-1) | N:P |
|---|---|---|---|
| Control | 37.05 ± 0.50 | 5.08 ± 0.07 a | 7.31 ± 0.13 c |
| N | 72.02 ± 1.15 a | 5.11 ± 0.05 a | 14.12 ± 0.25 a |
| P | 37.65 ± 0.64 b | 9.36 ± 0.11 b | 4.03 ± 0.07 d |
| NP | 71.91 ± 1.02 a | 9.18 ± 0.09 b | 7.84 ± 0.38 b |
表1 氮、磷添加及其交互作用影响的土壤有效氮、磷含量和有效氮磷比例(平均值±标准误)
Table 1 Effect of nitrogen (N) and phosphorus (P) addition and their interaction on available N and P concentration and available N:P ratio in bulk soil (mean ± SE)
| 处理 Treatment | 有效氮 Available N (mg·kg-1) | 有效磷 Available P (mg·kg-1) | N:P |
|---|---|---|---|
| Control | 37.05 ± 0.50 | 5.08 ± 0.07 a | 7.31 ± 0.13 c |
| N | 72.02 ± 1.15 a | 5.11 ± 0.05 a | 14.12 ± 0.25 a |
| P | 37.65 ± 0.64 b | 9.36 ± 0.11 b | 4.03 ± 0.07 d |
| NP | 71.91 ± 1.02 a | 9.18 ± 0.09 b | 7.84 ± 0.38 b |
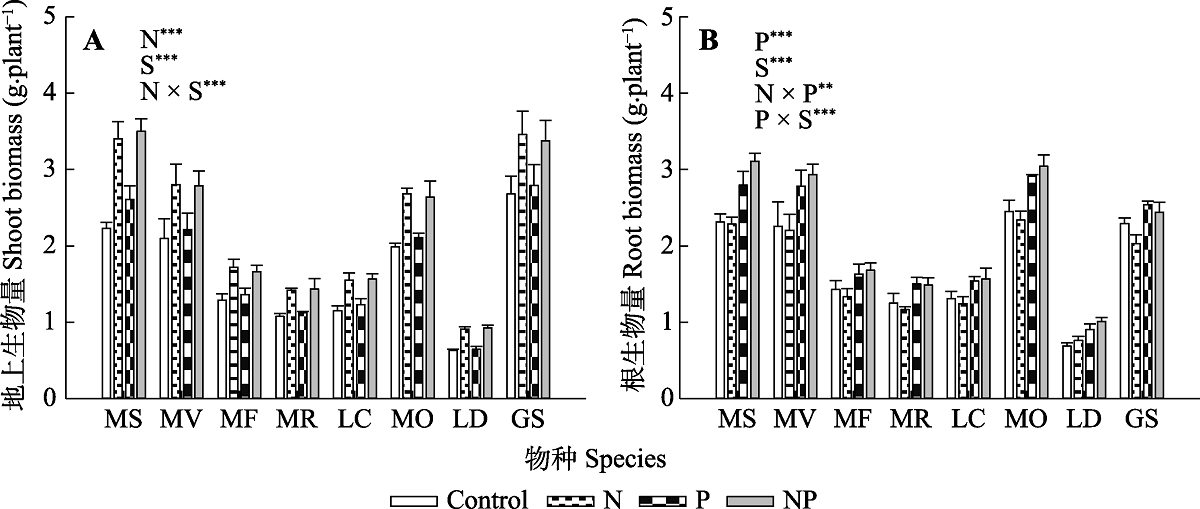
图1 氮、磷添加及其交互作用对不同豆科草本植物地上生物量(A)和根生物量(B)(平均值±标准误)的影响。Control, 无养分添加; N, 氮添加; P, 磷添加; NP, 氮磷耦合添加; S, 物种。GS, 野大豆; LC, 百脉根; LD, 兴安胡枝子; MF, 黄花苜蓿; MO, 草木樨; MR, 花苜蓿; MS, 紫花苜蓿; MV, 杂花苜蓿。星号表示经一般线性模型统计分析得因子效应具有显著性。***, p < 0.001; **, p < 0.01; *, p < 0.05。
Fig. 1 Influence of nitrogen (N) and phosphorus (P) addition and their interaction on shoot biomass (A) and root biomass (B)(mean ± SE) of different herbaceous legumes. Control, no nutrient addition; N, N addition; P, P addition; NP, coupled addition of N and P; S, species. GS, Glycine soja; LC, Lotus corniculatus; LD, Lespedeza daurica; MF, Medicago falcata; MO, Melilotus officinalis; MR, Medicago ruthenica; MS, Medicago sativa; MV, Medicago varia. Asterisks indicate that factor effect was significant by general linear model analysis. ***, p < 0.001; **, p < 0.01; *, p < 0.05.
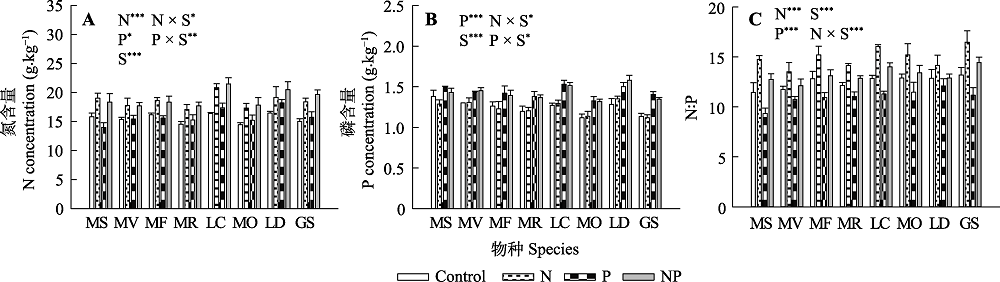
图2 氮、磷添加及其交互作用对不同豆科草本植物氮含量(A)、磷含量(B)和氮磷比(C)(平均值±标准误)的影响。Control, 无养分添加; N, 氮添加; P, 磷添加; NP, 氮磷耦合添加; S, 物种。GS, 野大豆; LC, 百脉根; LD, 兴安胡枝子; MF, 黄花苜蓿; MO, 草木樨; MR, 花苜蓿; MS, 紫花苜蓿; MV, 杂花苜蓿。星号表示经一般线性模型统计分析得因子效应具有显著性。***, p < 0.001; **, p < 0.01; *, p < 0.05。
Fig. 2 Influence of nitrogen (N) and phosphorus (P) addition and their interaction on plant N concentration (A), plant P concentration (B) and plant N:P ratio (C)(mean ± SE) of different herbaceous legumes. Control, no nutrient addition; N, N addition; P, P addition; NP, coupled addition of N and P; S, species. GS, Glycine soja; LC, Lotus corniculatus; LD, Lespedeza daurica; MF, Medicago falcata; MO, Melilotus officinalis; MR, Medicago ruthenica; MS, Medicago sativa; MV, Medicago varia. Asterisks indicate that factor effect was significant by general linear model analysis. ***, p < 0.001; **, p < 0.01; *, p < 0.05.
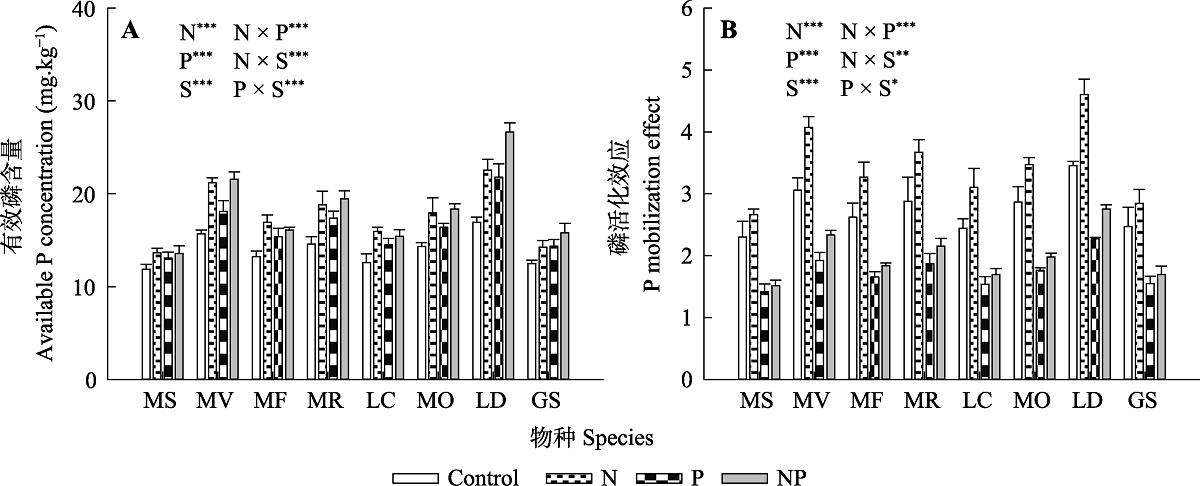
图3 氮、磷添加及其交互作用对不同豆科草本植物根际有效磷含量(A)和根际磷活化效应(B)(平均值±标准误)的影响。Control, 无养分添加; N, 氮添加; P, 磷添加; NP, 氮磷耦合添加; S, 物种。GS, 野大豆; LC, 百脉根; LD, 兴安胡枝子; MF, 黄花苜蓿; MO, 草木樨; MR, 花苜蓿; MS, 紫花苜蓿; MV, 杂花苜蓿。星号表示经一般线性模型统计分析得因子效应具有显著性。***, p < 0.001; **, p < 0.01; *, p < 0.05。
Fig. 3 Influence of nitrogen (N) and phosphorus (P) addition and their interaction on available P concentration in rhizosphere (A) and P mobilization in rhizosphere (B)(mean ± SE) of different herbaceous legumes. Control, no nutrient addition; N, N addition; P, P addition; NP, coupled addition of N and P; S, species. GS, Glycine soja; LC, Lotus corniculatus; LD, Lespedeza daurica; MF, Medicago falcata; MO, Melilotus officinalis; MR, Medicago ruthenica; MS, Medicago sativa; MV, Medicago varia. Asterisks indicate that factor effect was significant by general linear model analysis. ***, p < 0.001; **, p < 0.01; *, p < 0.05.
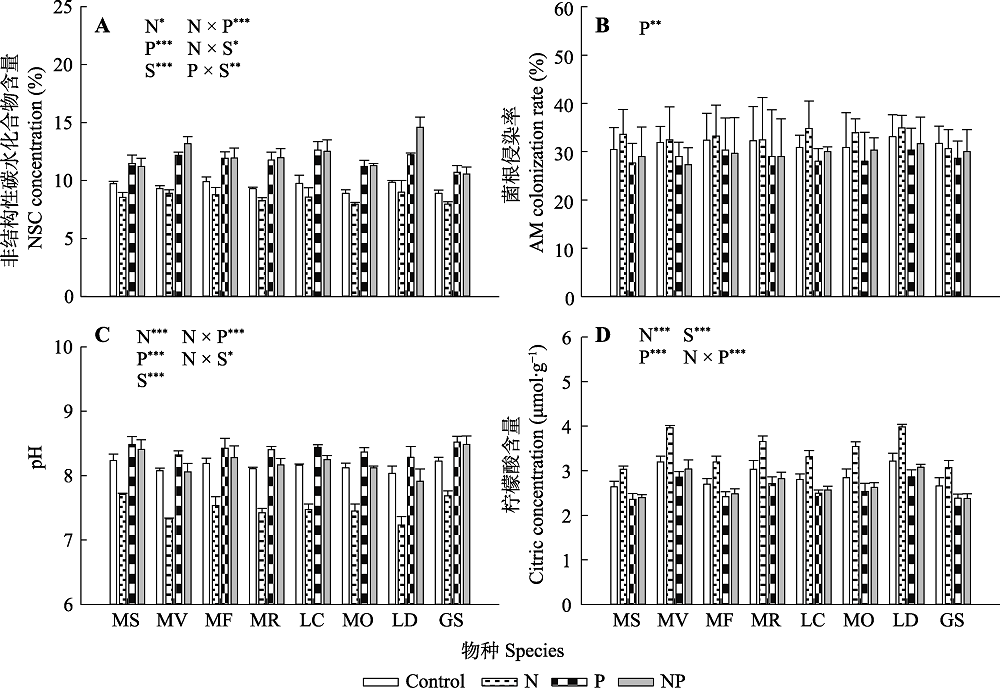
图4 氮、磷添加及其交互作用对不同豆科草本植物根系非结构性碳水化合物(NSC)含量(A)、根系的菌根(AM)侵染率(B)、根际pH (C)和根际柠檬酸含量(D)(平均值±标准误)的影响。Control, 无养分添加; N, 氮添加; P, 磷添加; NP, 氮磷耦合添加; S, 物种。GS, 野大豆; LC, 百脉根; LD, 兴安胡枝子; MF, 黄花苜蓿; MO, 草木樨; MR, 花苜蓿; MS, 紫花苜蓿; MV, 杂花苜蓿。星号表示经一般线性模型统计分析得因子效应具有显著性。***, p < 0.001; **, p < 0.01; *, p < 0.05。
Fig. 4 Influence of nitrogen (N) and phosphorus (P) addition and their interaction on non-structure carbohydrate (NSC) concentration in root (A), arbuscular mycorrhizal (AM) colonization rate of root (B), pH in rhizosphere (C) and citric concentration in rhizosphere (D)(mean ± SE) of different herbaceous legumes. Control, no nutrient addition; N, N addition; P, P addition; NP, coupled addition of N and P; S, species. GS, Glycine soja; LC, Lotus corniculatus; LD, Lespedeza daurica; MF, Medicago falcata; MO, Melilotus officinalis; MR, Medicago ruthenica; MS, Medicago sativa; MV, Medicago varia. Asterisks indicate that factor effect was significant by general linear model analysis. ***, p < 0.001; **, p < 0.01; *, p < 0.05.

图5 不同养分添加下8种豆科草本植物平均根生物量与根系菌根(AM)侵染率(A)、根际土壤pH (B)、根际柠檬酸含量(C)间相关关系, 及平均根系非结构性碳水化合物(NSC)含量与AM侵染率(D)、根际土壤pH (E)、根际柠檬酸含量(F)(平均值±标准误)间相关关系。
Fig. 5 Correlation relationships between mean root biomass and arbuscular mycorrhizal (AM) colonization rate of root (A), soil pH in rhizosphere (B), citric concentration in rhizosphere (C), and between mean non-structure carbohydrate (NSC) concentration in root and AM colonization rate of root (D), soil pH in rhizosphere (E), citric concentration in rhizosphere (F)(mean ± SE) following different nutrient addition treatments on eight herbaceous legumes.
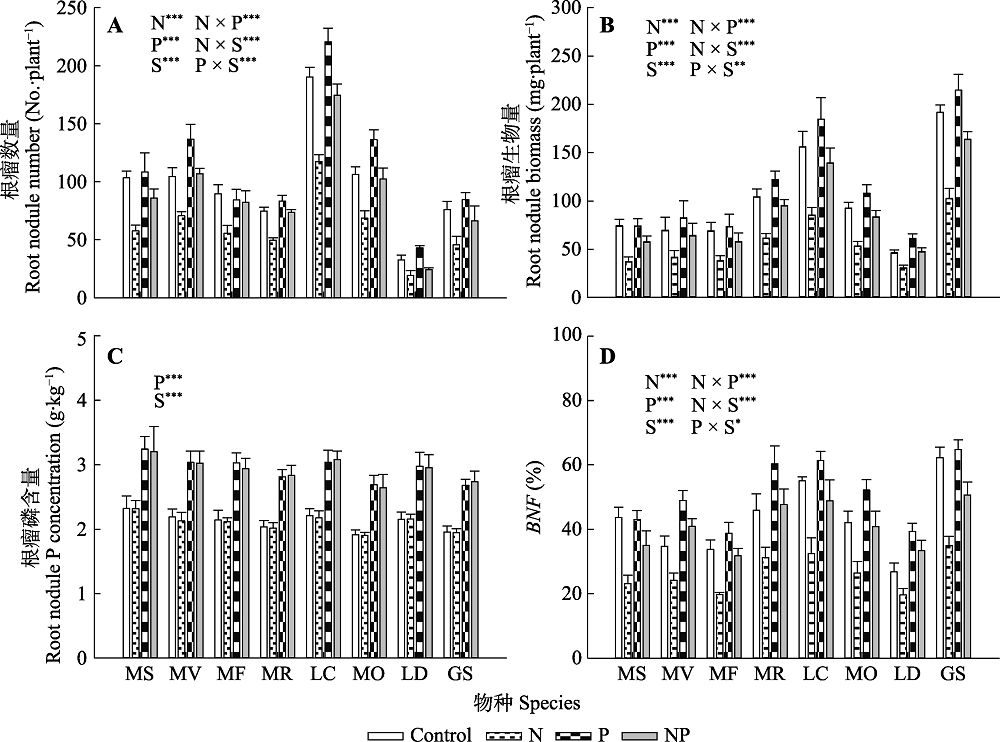
图6 氮、磷添加及其交互作用对不同豆科草本植物根瘤数目(A)、根瘤生物量(B)、根瘤磷含量(C)和生物固氮率(BNF)(D)(平均值±标准误)的影响。Control, 无养分添加; N, 氮添加; P, 磷添加; NP, 氮磷耦合添加; S, 物种。GS, 野大豆; LC, 百脉根; LD, 兴安胡枝子; MF, 黄花苜蓿; MO, 草木樨; MR, 花苜蓿; MS, 紫花苜蓿; MV, 杂花苜蓿。星号表示经一般线性模型统计分析得因子效应具有显著性。***, p < 0.001; **, p < 0.01; *, p < 0.05。
Fig. 6 Influence of nitrogen (N) and phosphorus (P) addition and their interaction on root nodule number (A), root nodule biomass (B), root nodule P concentration (C) and biological N fixation rate (BNF)(D)(mean ± SE) of different herbaceous legumes. Control, no nutrient addition; N, N addition; P, P addition; NP, coupled addition of N and P; S, species. GS, Glycine soja; LC, Lotus corniculatus; LD, Lespedeza daurica; MF, Medicago falcata; MO, Melilotus officinalis; MR, Medicago ruthenica; MS, Medicago sativa; MV, Medicago varia. Asterisks indicate that factor effect was significant by general linear model analysis. ***, p < 0.001; **, p < 0.01; *, p < 0.05.
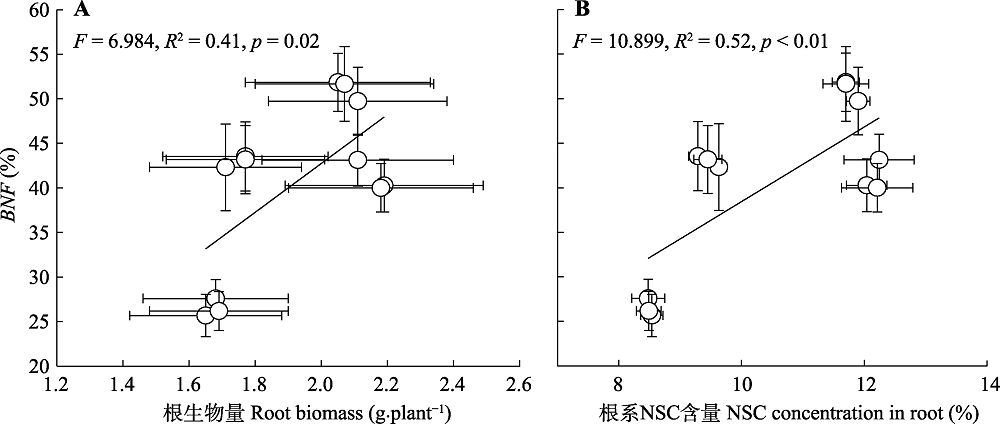
图7 不同养分添加下8种豆科草本植物平均根生物量(A)、根非结构性碳水化合物(NSC)含量(B)与豆科草本植物生物固氮率(BNF)(平均值±标准误)间相关关系。
Fig. 7 Correlation relationships between root biomass (A), root non-structure carbohydrate (NSC) concentration (B) and biological nitrogen fixation of legume (BNF)(mean ± SE) following different nutrient addition treatments on eight herbaceous legumes.
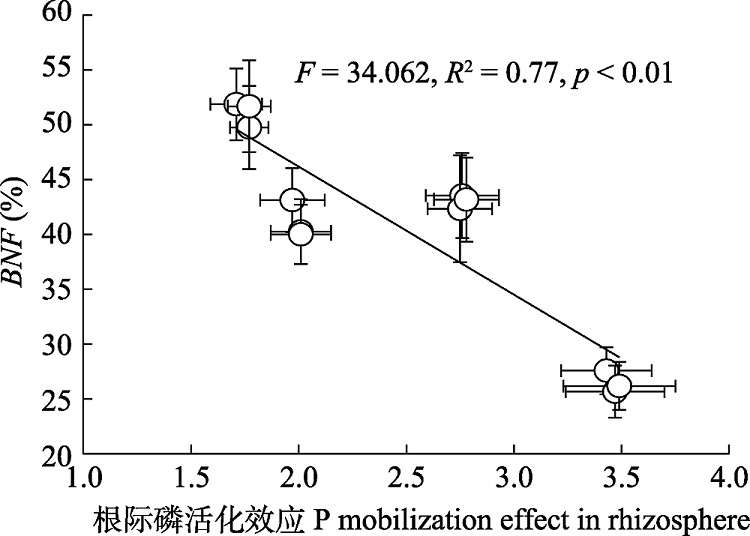
图8 不同养分添加下8种豆科草本植物平均根际磷活化效应与豆科草本植物生物固氮率(BNF)(平均值±标准误)间相关关系。
Fig. 8 Correlation relationships between phosphorus (P) mobilization effect in rhizosphere and biological nitrogen fixation rate of legume (BNF)(mean ± SE) following different nutrient addition treatments on eight herbaceous legumes.
| [1] |
Aerts R, Boot RGA, van der Aart PJM (1991). The relation between above- and belowground biomass allocation patterns and competitive ability. Oecologia, 87, 551-559.
DOI PMID |
| [2] |
Augusto L, Delerue F, Gallet-Budynek A, Achat DL (2013). Global assessment of limitation to symbiotic nitrogen fixation by phosphorus availability in terrestrial ecosystems using a meta-analysis approach. Global Biogeochemical Cycles, 27, 804-815.
DOI URL |
| [3] |
Bai Y, Wu J, Clark CM, Naeem S, Pan Q, Huang J, Zhang L, Han XG (2010). Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from Inner Mongolia grasslands. Global Change Biology, 16, 358-372.
DOI URL |
| [4] |
Batterman SA, Hedin LO, van Breugel M, Ransijn J, Craven DJ, Hall JS (2013). Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature, 502, 224-227.
DOI PMID |
| [5] |
Baziramakenga R, Simard RR, Leroux GD (1995). Determination of organic acids in soil extracts by ion chromatography. Soil Biology & Biochemistry, 27, 349-356.
DOI URL |
| [6] |
Betencourt E, Duputel M, Colomb B, Desclaux D, Hinsinger P (2012). Intercropping promotes the ability of durum wheat and chickpea to increase rhizosphere phosphorus availability in a low P soil. Soil Biology & Biochemistry, 46, 181-190.
DOI URL |
| [7] |
Buysse J, Merckx R (1993). An improved colorimetric method to quantify sugar content of plant tissue. Journal of Experimental Botany, 44, 1627-1629.
DOI URL |
| [8] |
Divito GA, Sadras VO (2014). How do phosphorus, potassium and sulphur affect plant growth and biological nitrogen fixation in crop and pasture legumes? A meta-analysis. Field Crops Research, 156, 161-171.
DOI URL |
| [9] |
Farrer EC, Suding KN (2016). Teasing apart plant community responses to N enrichment: the roles of resource limitation, competition and soil microbes. Ecology Letters, 19, 1287-1296.
DOI PMID |
| [10] |
Galloway JN (1998). The global nitrogen cycle: changes and consequences. Environmental Pollution, 102, 15-24.
DOI URL |
| [11] |
Gates CT, Wilson JR (1974). The interaction of nitrogen and phosphorus on the growth, nutrient status and nodulation of Stylosanthes humilis H.B.K. (townsville stylo). Plant and Soil, 41, 325-333.
DOI URL |
| [12] |
Gentili F, Huss-Danell K (2003). Local and systemic effects of phosphorus and nitrogen on nodulation and nodule function in Alnus incana. Journal of Experimental Botany, 54, 2757-2767.
DOI URL |
| [13] |
Güsewell S (2004). N:P ratios in terrestrial plants: variation and functional significance. New Phytologist, 164, 243-266.
DOI URL |
| [14] | Hinsinger P (1998). How do plant roots acquire mineral nutrients? Chemical processes involved in the rhizosphere. Advances in Agronomy, 64, 255-265. |
| [15] |
Hinsinger P, Plassard C, Tang C, Jaillard B (2003). Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant and Soil, 248, 43-59.
DOI URL |
| [16] |
Ledgard SF, Sprosen MS, Penno JW, Rajendram GS (2001). Nitrogen fixation by white clover in pastures grazed by dairy cows: temporal variation and effects of nitrogen fertilization. Plant and Soil, 229, 177-187.
DOI URL |
| [17] |
Ledgard SF, Steele KW (1992). Biological nitrogen fixation in mixed legume/grass pastures. Plant and Soil, 141, 137-153.
DOI URL |
| [18] |
Li Q, Song Y, Li G, Yu P, Wang P, Zhou D (2015). Grass-legume mixtures impact soil N, species recruitment, and productivity in temperate steppe grassland. Plant and Soil, 394, 271-285.
DOI URL |
| [19] |
Li Q, Yu P, Li G, Zhou D (2016a). Grass-legume ratio can change soil carbon and nitrogen storage in a temperate steppe grassland. Soil and Tillage Research, 157, 23-31.
DOI URL |
| [20] |
Li Q, Zhang H, Huang Y, Zhou D (2020). Forage nitrogen yield and soil nitrogen in artificial grasslands with varied Medicago seedling proportion. Archives of Agronomy and Soil Science, 66, 110-125.
DOI URL |
| [21] |
Li Y, Niu S, Yu G (2016b). Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: a meta-analysis. Global Change Biology, 22, 934-943.
DOI URL |
| [22] |
Mahowald N, Jickells TD, Baker AR, Artaxo P, Benitez-Nelson CR, Bergametti G, Bond TC, Chen Y, Cohen DD, Herut B, Kubilay N, Losno R, Luo C, Maenhaut W, McGee KA, Okin GS, Siefert RL, Tsukuda S (2008). Global distribution of atmospheric phosphorus sources, concentrations and deposition rates, and anthropogenic impacts. Global Biogeochemical Cycles, 22, GB4026. DOI: 10.1029/2008GB003240.
DOI |
| [23] |
Maistry PM, Cramer MD, Chimphango SBM (2013). N and P colimitation of N2-fixing and N-supplied fynbos legumes from the Cape Floristic Region. Plant and Soil, 373, 217-228.
DOI URL |
| [24] | Miller RH, Keeney DR (1982). Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties. 2nd ed. American Society of Agronomy, Madison, USA. |
| [25] |
Nuruzzaman M, Lambers H, Bolland MDA, Veneklaas EJ (2006). Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant and Soil, 281, 109-120.
DOI URL |
| [26] |
Nyfeler D, Huguenin-Elie O, Suter M, Frossard E, Lüscher A (2011). Grass-legume mixtures can yield more nitrogen than legume pure stands due to mutual stimulation of nitrogen uptake from symbiotic and non-symbiotic sources. Agriculture, Ecosystem & Environment, 140, 155-163.
DOI URL |
| [27] | Olsen SR, Cole CV, Watanabe SS, Dean LA (1954). Estimation of Available Phosphorus in Soil by Extraction with Sodium Bicarbonate. United States Department of Agriculture , Washington DC. |
| [28] |
Peñuelas J, Sardans J, Rivas-Ubach A, Janssens IA (2012). The human-induced imbalance between C, N and P in Earthʼs life system. Global Change Biology, 18, 3-6.
DOI URL |
| [29] |
Png GK, Turner BL, Albornoz FE, Hayes PE, Lambers H, Laliberté E (2017). Greater root phosphatase activity in nitrogen-fixing rhizobial but not actinorhizal plants with declining phosphorus availability. Journal of Ecology, 105, 1246-1255.
DOI URL |
| [30] |
Reed SC, Cleveland CC, Townsend AR (2007). Controls over leaf litter and soil nitrogen fixation in two lowland tropical rain forests. Biotropica, 39, 585-592.
DOI URL |
| [31] |
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C (2009). Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant and Soil, 321, 305-339.
DOI URL |
| [32] |
Salvagiotti F, Cassman KG, Specht JE, Walters DT, Weiss A, Dobermann A (2008). Nitrogen uptake, fixation and response to fertilizer N in soybeans: a review. Field Crops Research, 108, 1-13.
DOI URL |
| [33] |
Schade JD, Kyle M, Hobbie SE, Fagan WF, Elser JJ (2003). Stoichiometric tracking of soil nutrients by a desert insect herbivore. Ecology Letters, 6, 96-101.
DOI URL |
| [34] | Smith SE, Read D (2008). Mycorrhizal Symbiosis. 3rd ed. Academic Press, London, UK. |
| [35] |
Song YT, Zhou DW, Li Q, Wang P, Huang YX (2012). Leaf nitrogen and phosphorus stoichiometry in 80 herbaceous plant species of Songnen grassland in Northeast China. Chinese Journal of Plant Ecology, 36, 222-230.
DOI URL |
|
[宋彦涛, 周道玮, 李强, 王平, 黄迎新 (2012). 松嫩草地80种草本植物叶片氮磷化学计量特征. 植物生态学报, 36, 222-230.]
DOI |
|
| [36] |
Soper FM, Nasto MK, Osborne BB, Cleveland CC (2019). Nitrogen fixation and foliar nitrogen do not predict phosphorus acquisition strategies in tropical trees. Journal of Ecology, 107, 118-126.
DOI URL |
| [37] | Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (1996). Methods of Soil Analysis. Part 3. Chemical Methods. Soil Science Society of America, Madison, USA. |
| [38] |
Sulieman S, van Ha C, Schulze J, Phan Tran LS (2013). Growth and nodulation of symbiotic Medicago truncatula at different levels of phosphorus availability. Journal of Experimental Botany, 64, 2701-2712.
DOI PMID |
| [39] | Unkovich M, Herridge D, Peoples M, Cadisch G, Boddey B, Giller K, Alves B, Chalk P (2008). Measuring Plant- associated Nitrogen Fixation in Agricultural Systems. Australian Centre for International Agricultural Research, Canberra, Australia. |
| [40] | Vitousek PM, Cassman K, Cleveland C, Crews T, Field CB, Grimm NB, Howarth RW, Marino R, Martinelli L, Rastetter EB, Sprent JI (2002). Towards an ecological understanding of biological nitrogen fixation//Boyer EW, Howarth RW. The Nitrogen Cycle at Regional to Global Scales. Kluwer Academic Publishers, Dordrecht, the Netherlands.1-45. |
| [41] |
Vitousek PM, Menge DNL, Reed SC, Cleveland CC (2013). Biological nitrogen fixation: rates, patterns and ecological controls in terrestrial ecosystems. Philosophical Transactions of the Royal Society B: Biological Sciences, 368, 20130119. DOI: 10.1098/rstb.2013.0119.
DOI URL |
| [42] |
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010). Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecological Applications, 20, 5-15.
DOI URL |
| [43] |
West JB, HilleRisLambers J, Lee TD, Hobbie SE, Reich PB (2005). Legume species identity and soil nitrogen supply determine symbiotic nitrogen-fixation responses to elevated atmospheric [CO2]. New Phytologist, 167, 523-530.
DOI URL |
| [44] | White PJ, Hammond JP (2008). Phosphorus nutrition of terrestrial plants//White PJ, Hammond JP. The Ecophysiology of Plant-Phosphorus Interactions. Springer, Dordrecht, the Netherlands. 51-81. |
| [45] |
Wu N, Li Z, Liu H, Tang M (2015). Influence of arbuscular mycorrhiza on photosynthesis and water status of Populus cathayana Rehder males and females under salt stress. Acta Physiol Plant, 37, 183.
DOI URL |
| [46] |
Zhan S, Wang Y, Zhu Z, Li W, Bai Y (2017). Nitrogen enrichment alters plant N:P stoichiometry and intensifies phosphorus limitation in a steppe ecosystem. Environmental and Experimental Botany, 134, 21-32.
DOI URL |
| [47] |
Zheng M, Zhou Z, Luo Y, Zhao P, Mo J (2019). Global pattern and controls of biological nitrogen fixation under nutrient enrichment: a meta-analysis. Global Change Biology, 25, 3018-3030.
DOI URL |
| [48] |
Zhou LL, Cao J, Zhang FS, Li L (2009). Rhizosphere acidification of faba bean, soybean and maize. Science of the Total Environment, 407, 4356-4362.
DOI URL |
| [49] |
Zou CM, Wang YQ, Liu Y, Zhang XH, Tang S (2015). Responses of photosynthesis and growth to weak light regime in four legume species. Chinese Journal of Plant Ecology, 39, 909-916.
DOI URL |
|
[邹长明, 王允青, 刘英, 张晓红, 唐杉 (2015). 四种豆科作物的光合生理和生长发育对弱光的响应. 植物生态学报, 39, 909-916.]
DOI |
| [1] | 刘瑶 钟全林 徐朝斌 程栋梁 郑跃芳 邹宇星 张雪 郑新杰 周云若. 不同大小刨花楠细根功能性状与根际微环境关系[J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [2] | 付粱晨, 丁宗巨, 唐茂, 曾辉, 朱彪. 北京东灵山白桦和蒙古栎的根际效应及其季节动态[J]. 植物生态学报, 2024, 48(4): 508-522. |
| [3] | 张英, 张常洪, 汪其同, 朱晓敏, 尹华军. 氮沉降下西南山地针叶林根际和非根际土壤固碳贡献差异[J]. 植物生态学报, 2023, 47(9): 1234-1244. |
| [4] | 张仲富, 王四海, 杨卫, 陈剑. 蒜头果根际细菌群落结构与功能特征对其健康状态的响应[J]. 植物生态学报, 2023, 47(7): 1020-1031. |
| [5] | 李柳, 刘庆华, 尹春英. 植物硒生物强化及微生物在其中的应用潜力[J]. 植物生态学报, 2023, 47(6): 756-769. |
| [6] | 张尧, 陈岚, 王洁莹, 李益, 王俊, 郭垚鑫, 任成杰, 白红英, 孙昊田, 赵发珠. 太白山不同海拔森林根际土壤微生物碳利用效率差异性及其影响因素[J]. 植物生态学报, 2023, 47(2): 275-288. |
| [7] | 张英, 张常洪, 汪其同, 朱晓敏, 尹华军. 氮沉降下西南山地针叶林根际和非根际土壤微生物养分限制特征差异[J]. 植物生态学报, 2022, 46(4): 473-483. |
| [8] | 李孝龙, 周俊, 彭飞, 钟宏韬, Hans LAMBERS. 植物养分捕获策略随成土年龄的变化及生态学意义[J]. 植物生态学报, 2021, 45(7): 714-727. |
| [9] | 王银柳, 耿倩倩, 黄建辉, 王常慧, 李磊, 哈斯木其尔, 牛国祥. 氮肥和种植密度对达乌里胡枝子的生长与生物固氮的影响[J]. 植物生态学报, 2021, 45(1): 13-22. |
| [10] | 胡琪娟, 盛茂银, 殷婕, 白义鑫. 西南喀斯特石漠化环境适生植物构树细根、根际土壤化学计量特征[J]. 植物生态学报, 2020, 44(9): 962-972. |
| [11] | 扈明媛, 袁野, 戴晓琴, 付晓莉, 寇亮, 王辉民. 亚热带人工林乔灌草根际土壤氮矿化特征[J]. 植物生态学报, 2020, 44(12): 1285-1295. |
| [12] | 崔利, 郭峰, 张佳蕾, 杨莎, 王建国, 孟静静, 耿耘, 李新国, 万书波. 摩西斗管囊霉改善连作花生根际土壤的微环境[J]. 植物生态学报, 2019, 43(8): 718-728. |
| [13] | 闫雅楠, 叶小齐, 吴明, 闫明, 张昕丽. 入侵植物加拿大一枝黄花根际解钾菌多样性及解钾活性[J]. 植物生态学报, 2019, 43(6): 543-556. |
| [14] | 范紫腾, 毋钰灵, 王新菊, 李太强, 高江云. 共生真菌对兰科植物种间杂交后代种子萌发的效应[J]. 植物生态学报, 2019, 43(4): 374-382. |
| [15] | 邹瓒, 陈劲松, 李洋, 宋会兴. 光合产物传输方向对蓉城竹根际微生物过程的影响[J]. 植物生态学报, 2018, 42(8): 863-872. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19