

植物生态学报 ›› 2014, Vol. 38 ›› Issue (12): 1345-1355.DOI: 10.3724/SP.J.1258.2014.00129
收稿日期:2014-04-03
接受日期:2014-08-18
出版日期:2014-04-03
发布日期:2015-04-16
通讯作者:
万贤崇
作者简介:* (E-mail: wxc@caf.ac.cn)
ZHANG Cui-Ping1, MENG Ping2, LI Jian-Zhong3, WAN Xian-Chong1,*( )
)
Received:2014-04-03
Accepted:2014-08-18
Online:2014-04-03
Published:2015-04-16
Contact:
WAN Xian-Chong
摘要:
核桃(Juglans regia)向南推广种植不可避免地会遇到土壤酸化和缺磷的环境, 这种环境如何影响核桃的生长是生产中需要知晓的基础问题。该文研究了土壤不同pH值对核桃的磷素营养影响以及缺磷对核桃幼苗水分平衡、光合特性和生长的影响。在温室内采用砂培盆栽试验, 研究一年生核桃嫁接幼苗在不同pH值、磷水平基质中的水分关系、光合特性和生长的应对机制。研究设4种处理, 即: 对照(正常供应磷素+ pH 6.0); 正常供应磷素+ pH 3.0; 不添加磷素+ pH 6.0; 不添加磷素+ pH 3.0。结果显示: pH值与磷素对核桃幼苗的影响是两个相互独立的过程, 酸性(pH值3.0)条件下, 核桃幼苗根系生物量降低、根冠比减小, 根系导水率降低, 对磷素的吸收利用减少, 尽管其供磷正常, 但各生长指标及生理指标与磷胁迫条件下反应相似; 两因素具有一定的叠加性, 在磷胁迫条件下, 酸化(pH值3.0)对核桃幼苗的损害进一步加剧。各指标具体变化如下: 酸化及磷胁迫条件下核桃根系水分导度降低, 叶柄木质部结构改变, 导管密度降低, 木质部导管栓塞程度增加, 叶柄导水率下降, 植株水分运输效率降低, 叶片水势降低, 诱导气孔关闭; 气孔导度降低, 光合作用能力下降; 胁迫条件下, 叶绿素荧光参数最大光化学效率低于0.8, 实际光化学效率、光化学淬灭下降, 非光化学淬灭增加, 核桃幼苗受胁迫环境损害, 叶片光系统II光合电子传递活性受到抑制, 光合能力下降。总之, 土壤酸化抑制了核桃幼苗对磷元素的吸收利用, 造成体内缺磷; 磷胁迫及酸化抑制了叶柄木质部的发育, 降低了根系水分导度和叶柄导水率, 干扰了核桃幼苗水分平衡, 通过气孔与非气孔共同调节, 限制了核桃幼苗光合作用, 抑制了核桃幼苗高生长、直径生长及叶面积增加; 但并没有发现土壤酸化和缺磷之间有明显的交互作用。
张翠萍, 孟平, 李建中, 万贤崇. 磷元素和土壤酸化交互作用对核桃幼苗光合特性的影响. 植物生态学报, 2014, 38(12): 1345-1355. DOI: 10.3724/SP.J.1258.2014.00129
ZHANG Cui-Ping, MENG Ping, LI Jian-Zhong, WAN Xian-Chong. Interactive effects of soil acidification and phosphorus deficiency on photosynthetic characteristics and growth in Juglans regia seedlings. Chinese Journal of Plant Ecology, 2014, 38(12): 1345-1355. DOI: 10.3724/SP.J.1258.2014.00129
| 营养元素 Nutrition | 缺磷 Phosphorus deficiency | 正常磷 Normal phosphorus |
|---|---|---|
| KNO3 | 4 | 2 |
| K2HPO4 | - | 1 |
| NH4NO3 | 1 | 2 |
| (NH4)2SO4 | 3 | 3 |
| K2SO4 | - | - |
| MgSO4 | 2 | 2 |
| KH2PO4 | - | - |
| Ca(NO3)2 | 2 | 2 |
表1 磷处理营养液成分
Table 1 Composition of nutrient solution under different phosphorus treatments (mmol·L-1)
| 营养元素 Nutrition | 缺磷 Phosphorus deficiency | 正常磷 Normal phosphorus |
|---|---|---|
| KNO3 | 4 | 2 |
| K2HPO4 | - | 1 |
| NH4NO3 | 1 | 2 |
| (NH4)2SO4 | 3 | 3 |
| K2SO4 | - | - |
| MgSO4 | 2 | 2 |
| KH2PO4 | - | - |
| Ca(NO3)2 | 2 | 2 |
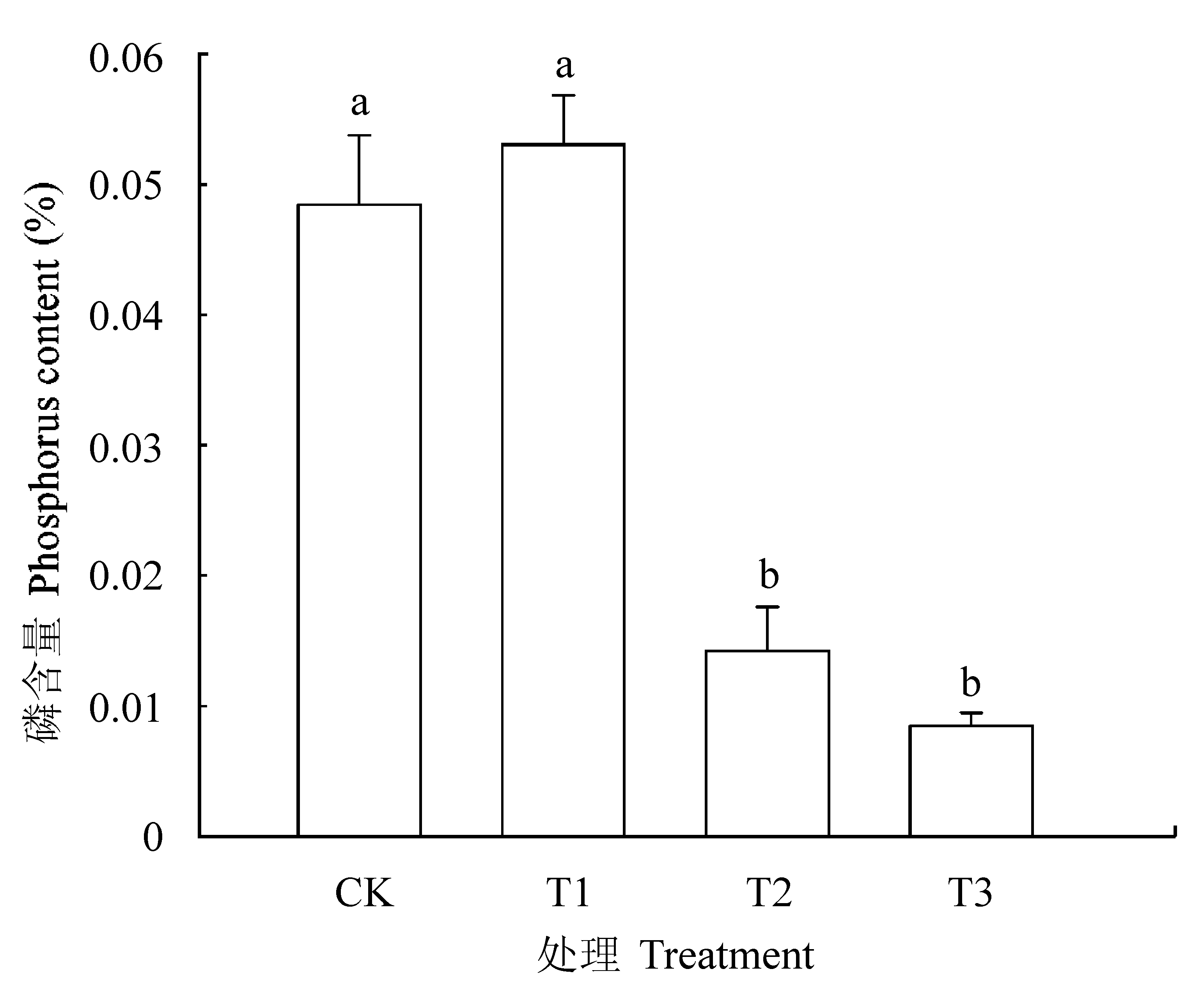
图1 土壤磷含量(平均值±标准误差)。 CK, 正常供磷素+ pH 6.0; T1, 正常供磷素+ pH 3.0; T2, 不添加磷素+ pH 6.0; T3, 不添加磷素+ pH 3.0。 不同小写字母表示不同处理间差异显著(p < 0.05)。
Fig. 1 Phosphorus content in soil (mean ± SE). CK, normal phosphorus and pH 6.0; T1, normal phosphorus and pH 3.0; T2, phosphorus deficiency and pH 6.0; T3, phosphorus deficiency and pH 3.0. Different small letters indicate significant differences among treatments (p < 0.05).
| 土壤 Soil | 根 Root | 叶片 Leaf | 茎 Shoot | 全株 Total plant | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | |||||
| Phosphorus (P) | 324.527 | 0.000 | 121.359 | 0.000 | 188.985 | 0.000 | 57.784 | 0.000 | 431.250 | 0.000 | ||||
| pH | 0.127 | 0.731 | 33.458 | 0.000 | 129.631 | 0.000 | 28.593 | 0.001 | 202.247 | 0.000 | ||||
| P × pH | 5.428 | 0.055 | 2.314 | 0.167 | 56.035 | 0.000 | 6.718 | 0.032 | 24.811 | 0.001 | ||||
表2 磷元素与pH值对土壤及核桃幼苗各器官磷含量影响的双因素分析
Table 2 Two-way ANOVA for testing the effects of phosphorus and pH value on phosphorus content in soil and seedlings
| 土壤 Soil | 根 Root | 叶片 Leaf | 茎 Shoot | 全株 Total plant | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | |||||
| Phosphorus (P) | 324.527 | 0.000 | 121.359 | 0.000 | 188.985 | 0.000 | 57.784 | 0.000 | 431.250 | 0.000 | ||||
| pH | 0.127 | 0.731 | 33.458 | 0.000 | 129.631 | 0.000 | 28.593 | 0.001 | 202.247 | 0.000 | ||||
| P × pH | 5.428 | 0.055 | 2.314 | 0.167 | 56.035 | 0.000 | 6.718 | 0.032 | 24.811 | 0.001 | ||||
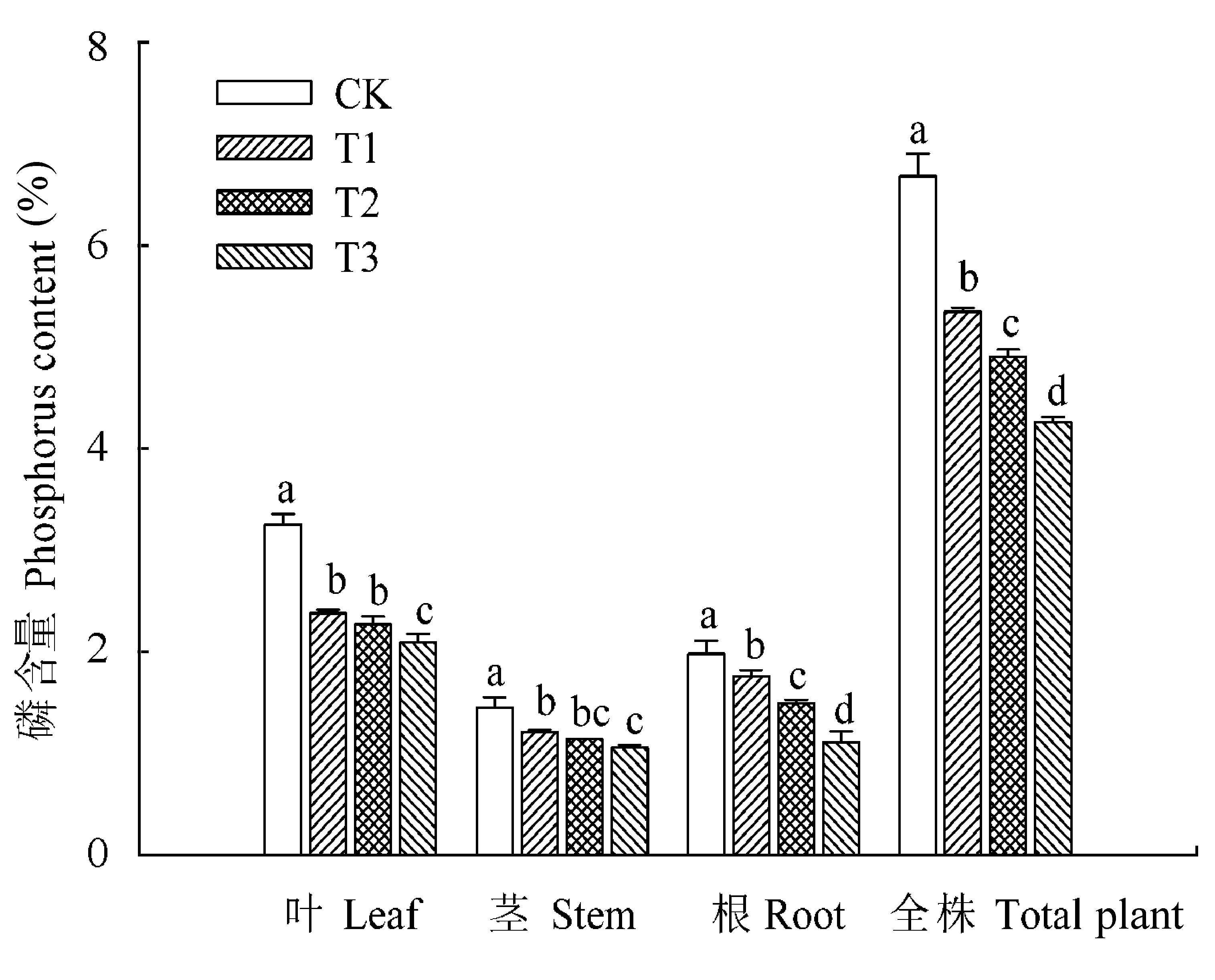
图2 核桃各器官中磷含量(平均值±标准误差)。CK, 正常供磷素+ pH 6.0; T1, 正常供磷素+ pH 3.0; T2, 不添加磷素+ pH 6.0; T3, 不添加磷素+ pH 3.0。 不同小写字母表示不同处理间差异显著(p < 0.05)。
Fig. 2 Phosphorus content in different organs of Juglans regia (mean ± SE). CK, normal phosphorus and pH 6.0; T1, normal phosphorus and pH 3.0; T2, phosphorus deficiency and pH 6.0; T3, phosphorus deficiency and pH 3.0. Different small letters indicate significant differences among treatments (p < 0.05).
| 生长指标 Growth variable | 处理 Treatment | |||
|---|---|---|---|---|
| CK | T1 | T2 | T3 | |
| 根生物量 Root biomass | 109.59b | 102.16c | 121.61a | 89.52d |
| 茎生物量 Shoot biomass | 33.36a | 29.27b | 26.33b | 22.12c |
| 叶生物量 Leaf biomass | 16.76a | 15.10a | 14.31a | 9.77a |
| 地下生物量/地上生物量 Root biomass / Shoot biomass | 2.19b | 2.31b | 3.01a | 2.83a |
| 高生长 Height increment (cm) | 10.23a | 9.18b | 8.68b | 7.68c |
| 茎生长 Diameter growth (mm) | 4.76a | 2.61b | 2.09bc | 1.69c |
| 叶面积 Leaf area (cm2) | 116.96a | 96.43b | 87.43bc | 82.82c |
表3 不同处理条件下核桃幼苗的生长与生物量分配
Table 3 Growth and biomass allocation in Juglans regia seedlings under different treatments
| 生长指标 Growth variable | 处理 Treatment | |||
|---|---|---|---|---|
| CK | T1 | T2 | T3 | |
| 根生物量 Root biomass | 109.59b | 102.16c | 121.61a | 89.52d |
| 茎生物量 Shoot biomass | 33.36a | 29.27b | 26.33b | 22.12c |
| 叶生物量 Leaf biomass | 16.76a | 15.10a | 14.31a | 9.77a |
| 地下生物量/地上生物量 Root biomass / Shoot biomass | 2.19b | 2.31b | 3.01a | 2.83a |
| 高生长 Height increment (cm) | 10.23a | 9.18b | 8.68b | 7.68c |
| 茎生长 Diameter growth (mm) | 4.76a | 2.61b | 2.09bc | 1.69c |
| 叶面积 Leaf area (cm2) | 116.96a | 96.43b | 87.43bc | 82.82c |
| 根生物量 Root biomass | 茎生物量 Shoot biomass | 叶生物量 Leaf biomass | 地下生物量/地上生物量 Root biomass / Shoot biomass | 高生长 Height increment (cm) | 茎生长 Diameter growth (mm) | 叶面积 Leaf area (cm2) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | |||||||
| Phosphorus (P) | 0.056 | 0.817 | 38.236 | 0.000 | 0.106 | 0.749 | 46.398 | 0.000 | 13.89 | 0.001 | 87.615 | 0.000 | 30.435 | 0.000 | ||||||
| pH | 224.433 | 0.000 | 13.113 | 0.002 | 0.108 | 0.747 | 0.085 | 0.775 | 9.360 | 0.004 | 45.942 | 0.000 | 8.775 | 0.004 | ||||||
| P × pH | 87.391 | 0.000 | 0.003 | 0.958 | 0.858 | 0.368 | 2.238 | 0.154 | 0.028 | 0.868 | 23.349 | 0.000 | 3.307 | 0.073 | ||||||
表4 磷元素与pH值对核桃幼苗生长与生物量分配影响的双因素分析
Table 4 Two-way ANOVA for testing the effects of phosphorus and pH value on growth and biomass allocation in Juglans regia seedlings
| 根生物量 Root biomass | 茎生物量 Shoot biomass | 叶生物量 Leaf biomass | 地下生物量/地上生物量 Root biomass / Shoot biomass | 高生长 Height increment (cm) | 茎生长 Diameter growth (mm) | 叶面积 Leaf area (cm2) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | |||||||
| Phosphorus (P) | 0.056 | 0.817 | 38.236 | 0.000 | 0.106 | 0.749 | 46.398 | 0.000 | 13.89 | 0.001 | 87.615 | 0.000 | 30.435 | 0.000 | ||||||
| pH | 224.433 | 0.000 | 13.113 | 0.002 | 0.108 | 0.747 | 0.085 | 0.775 | 9.360 | 0.004 | 45.942 | 0.000 | 8.775 | 0.004 | ||||||
| P × pH | 87.391 | 0.000 | 0.003 | 0.958 | 0.858 | 0.368 | 2.238 | 0.154 | 0.028 | 0.868 | 23.349 | 0.000 | 3.307 | 0.073 | ||||||
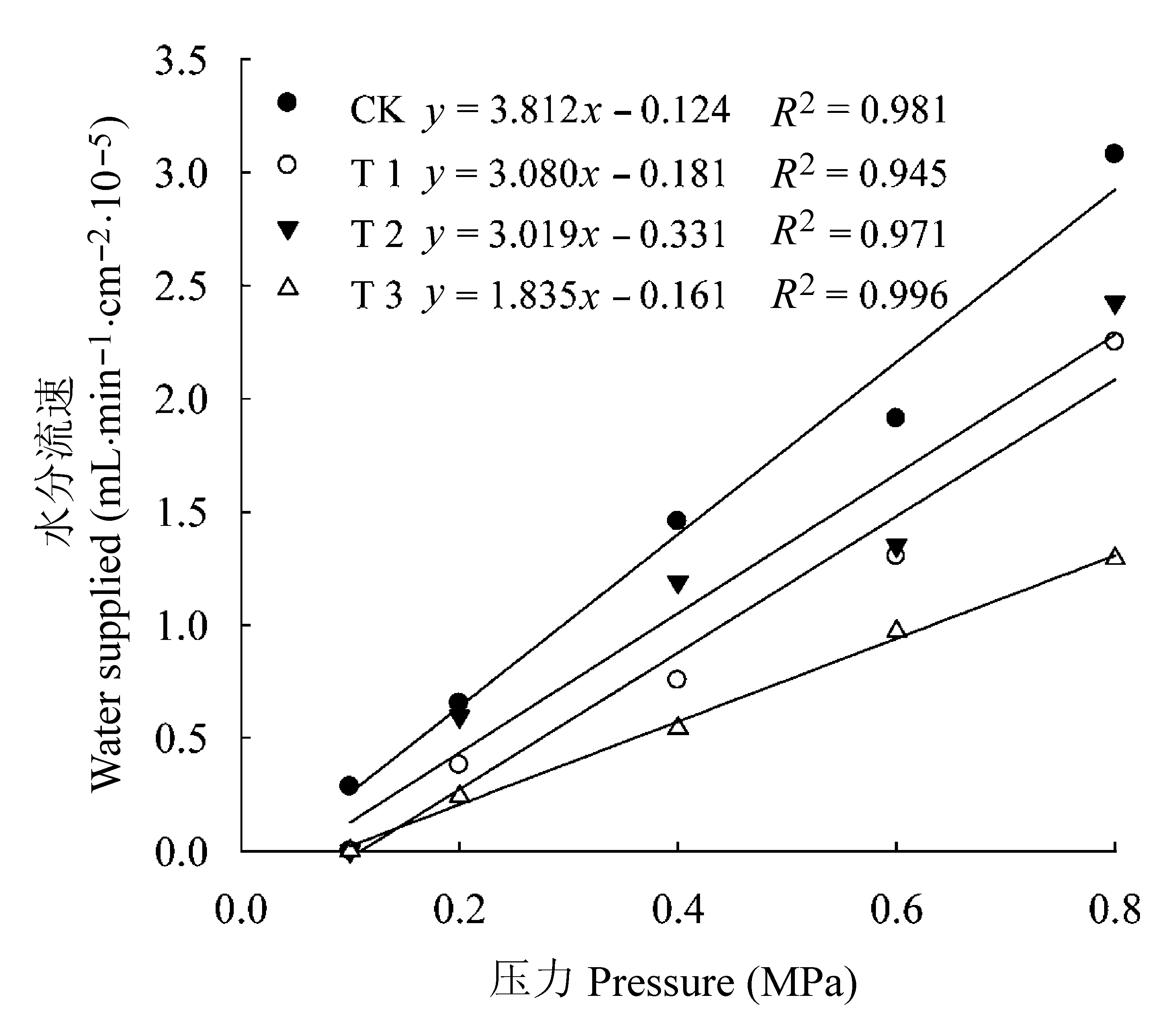
图3 各处理条件下核桃根系水分流速与压力差的关系。CK, 正常供磷素+ pH 6.0; T1, 正常供磷素+ pH 3.0; T2, 不添加磷素+ pH 6.0; T3, 不添加磷素+ pH 3.0。
Fig. 3 The relationships between pressure drop and water velocity in Juglans regia roots under different treatments. CK, normal phosphorus and pH 6.0; T1, normal phosphorus and pH 3.0; T2, phosphorus deficiency and pH 6.0; T3, phosphorus deficiency and pH 3.0.
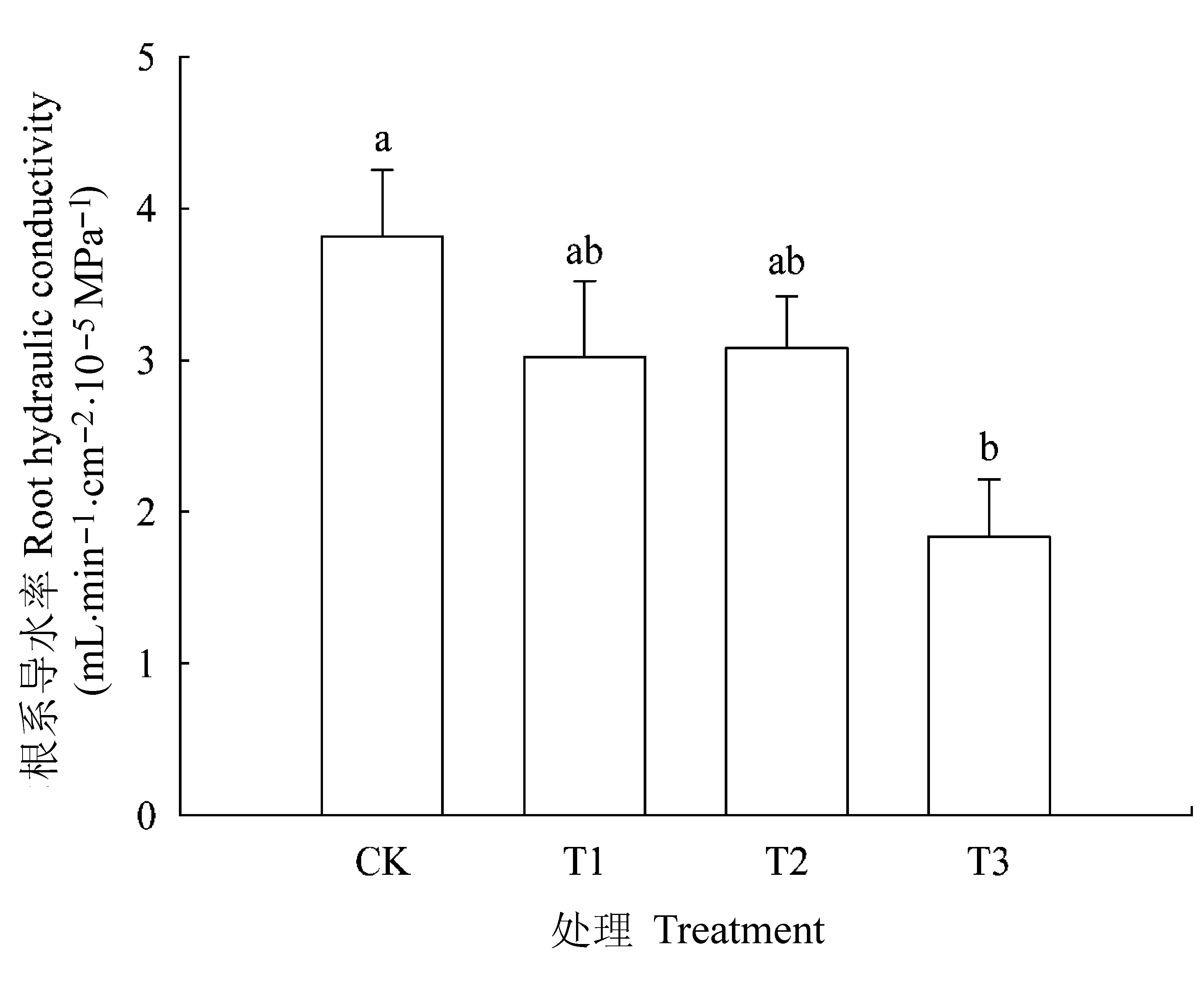
图4 不同处理条件下核桃幼苗的根系导水率(平均值±标准误差)。CK, 正常供磷素+ pH 6.0; T1, 正常供磷素+ pH 3.0; T2, 不添加磷素+ pH 6.0; T3, 不添加磷素+ pH 3.0。 不同小写字母表示不同处理间差异显著(p < 0.05)。
Fig. 4 Root hydraulic conductivity in Juglans regia seedlings under different treatments (mean ± SE). CK, normal phosphorus and pH 6.0; T1, normal phosphorus and pH 3.0; T2, phosphorus deficiency and pH 6.0; T3, phosphorus deficiency and pH 3.0. Different small letters indicate significant differences among treatments (p < 0.05).
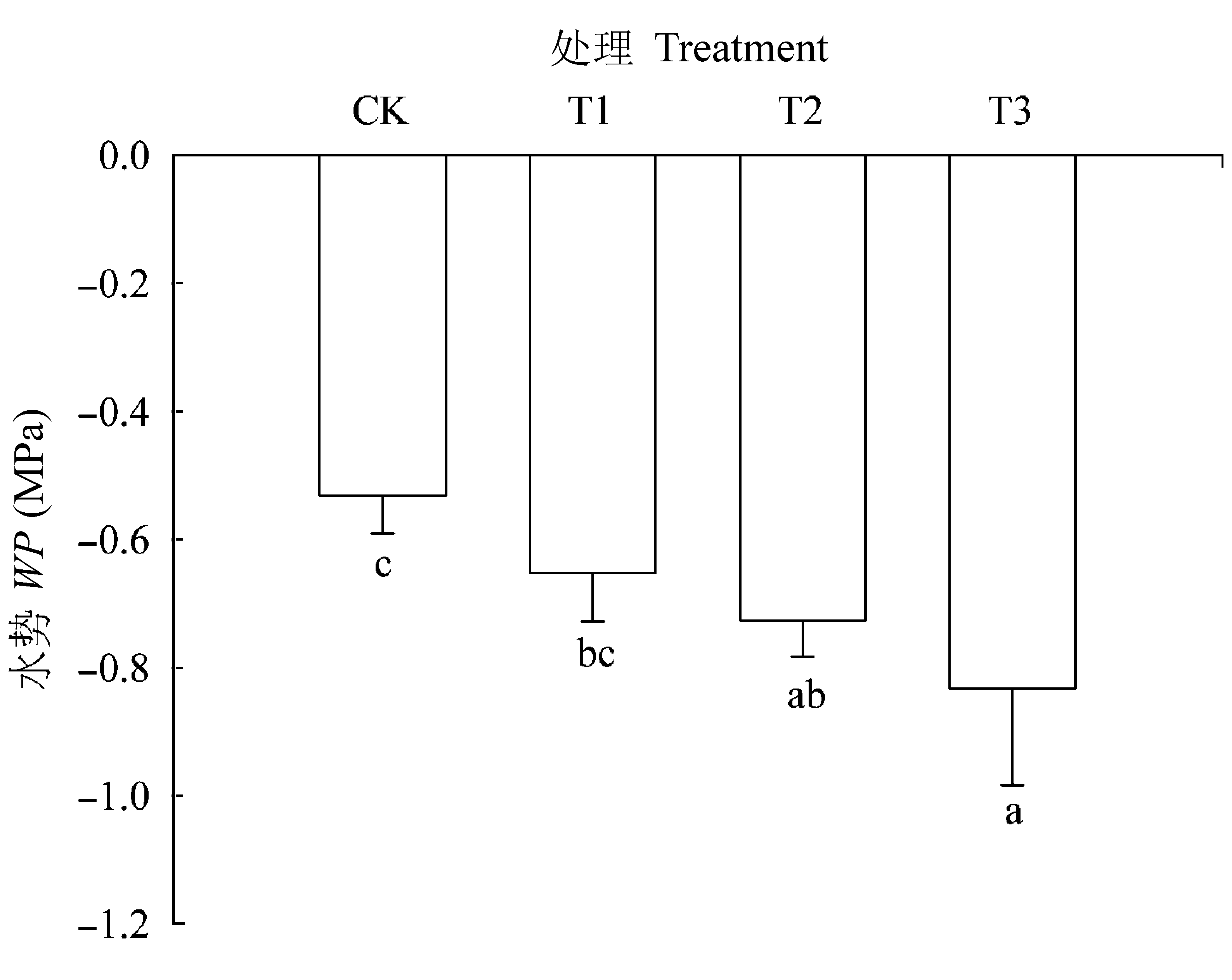
图5 不同处理条件下核桃叶片水势(平均值±标准误差)。CK, 正常供磷素+ pH 6.0; T1, 正常供磷素+ pH 3.0; T2, 不添加磷素+ pH 6.0; T3, 不添加磷素+ pH 3.0。 不同小写字母表示不同处理间差异显著(p < 0.05)。
Fig. 5 Leaf water potential (WP) in Juglans regia under different treatments (mean ± SE). CK, normal phosphorus and pH 6.0; T1, normal phosphorus and pH 3.0; T2, phosphorus deficiency and pH 6.0; T3, phosphorus deficiency and pH 3.0. Different small letters indicate significant differences among treatments (p < 0.05).
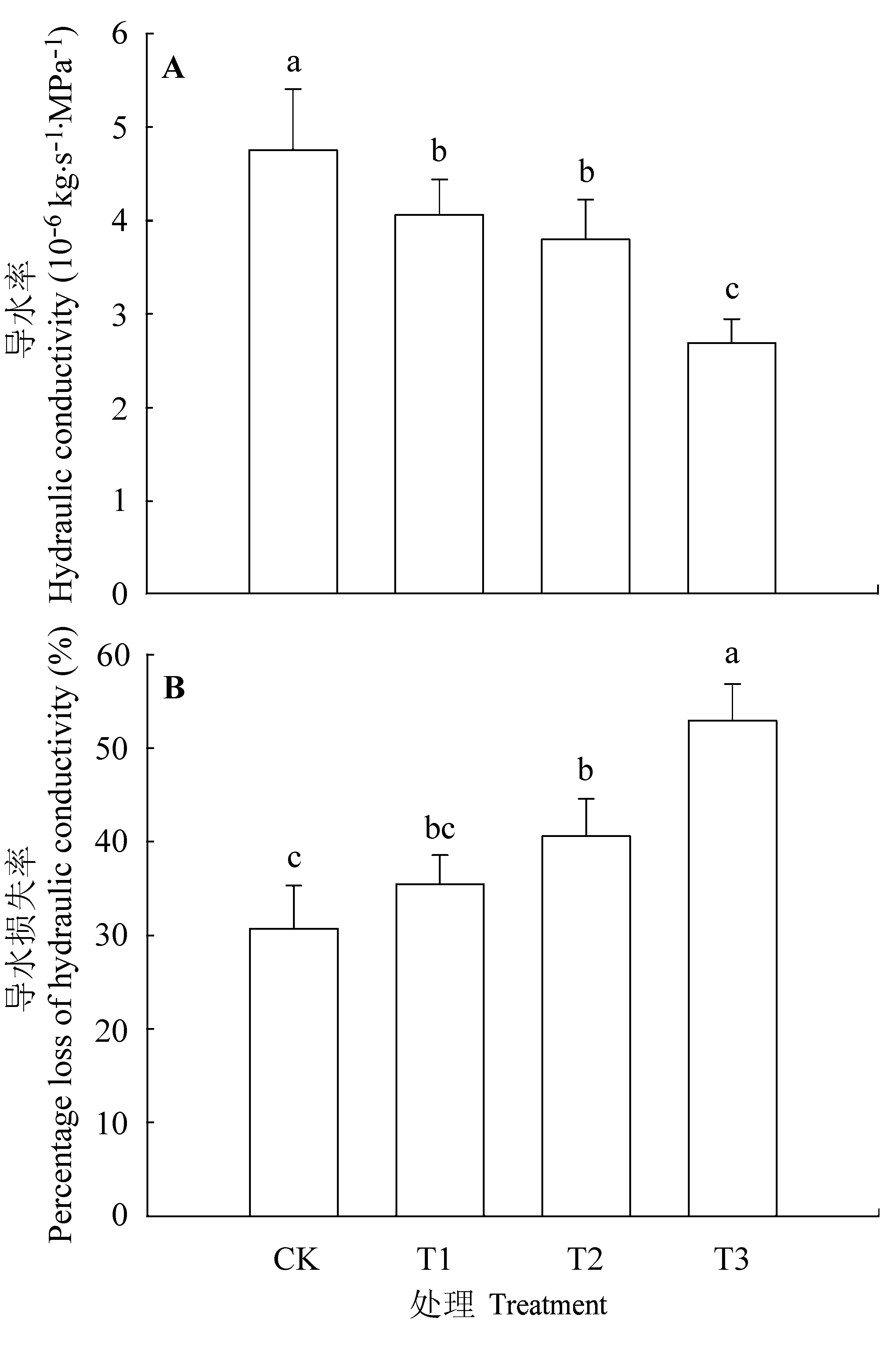
图6 不同处理条件下核桃叶柄导水率和导水损失率(平均值±标准误差)。CK, 正常供磷素+ pH 6.0; T1, 正常供磷素+ pH 3.0; T2, 不添加磷素+ pH 6.0; T3, 不添加磷素+ pH 3.0。 不同小写字母表示不同处理间差异显著(p < 0.05)。
Fig. 6 Hydraulic conductivity and percentage loss of hydraulic conductivity (PLC) in Juglans regia petioles under different treatments (mean ± SE). CK, normal phosphorus and pH 6.0; T1, normal phosphorus and pH 3.0; T2, phosphorus deficiency and pH 6.0; T3, phosphorus deficiency and pH 3.0. Different small letters indicate significant differences among treatments (p < 0.05).
| 根系导水率 Root hydraulic conductance | 导水损失率 Percentage loss of hydraulic conductance | 导水率 Hydraulic conductivity | 导管密度 Vessel density (No.·mm-2) | 导管直径 Vessel diameter (μm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | |||||
| Phosphorus (P) | 5.132 | 0.043 | 59.903 | 0.000 | 33.211 | 0.000 | 76.056 | 0.000 | 6.285 | 0.017 | ||||
| pH | 5.578 | 0.036 | 23.256 | 0.000 | 19.911 | 0.000 | 61.606 | 0.000 | 2.637 | 0.114 | ||||
| P × pH | 0.367 | 0.556 | 4.646 | 0.051 | 1.069 | 0.317 | 2.714 | 0.108 | 0.525 | 0.474 | ||||
表5 磷元素与pH值对核桃幼苗根系导水率及叶柄水分运输影响双因素分析
Table 5 Two-way ANOVA for testing the effects of phosphorus and pH value on root hydraulic conductivity and petiole water transport in Juglans regia seedlings
| 根系导水率 Root hydraulic conductance | 导水损失率 Percentage loss of hydraulic conductance | 导水率 Hydraulic conductivity | 导管密度 Vessel density (No.·mm-2) | 导管直径 Vessel diameter (μm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | |||||
| Phosphorus (P) | 5.132 | 0.043 | 59.903 | 0.000 | 33.211 | 0.000 | 76.056 | 0.000 | 6.285 | 0.017 | ||||
| pH | 5.578 | 0.036 | 23.256 | 0.000 | 19.911 | 0.000 | 61.606 | 0.000 | 2.637 | 0.114 | ||||
| P × pH | 0.367 | 0.556 | 4.646 | 0.051 | 1.069 | 0.317 | 2.714 | 0.108 | 0.525 | 0.474 | ||||
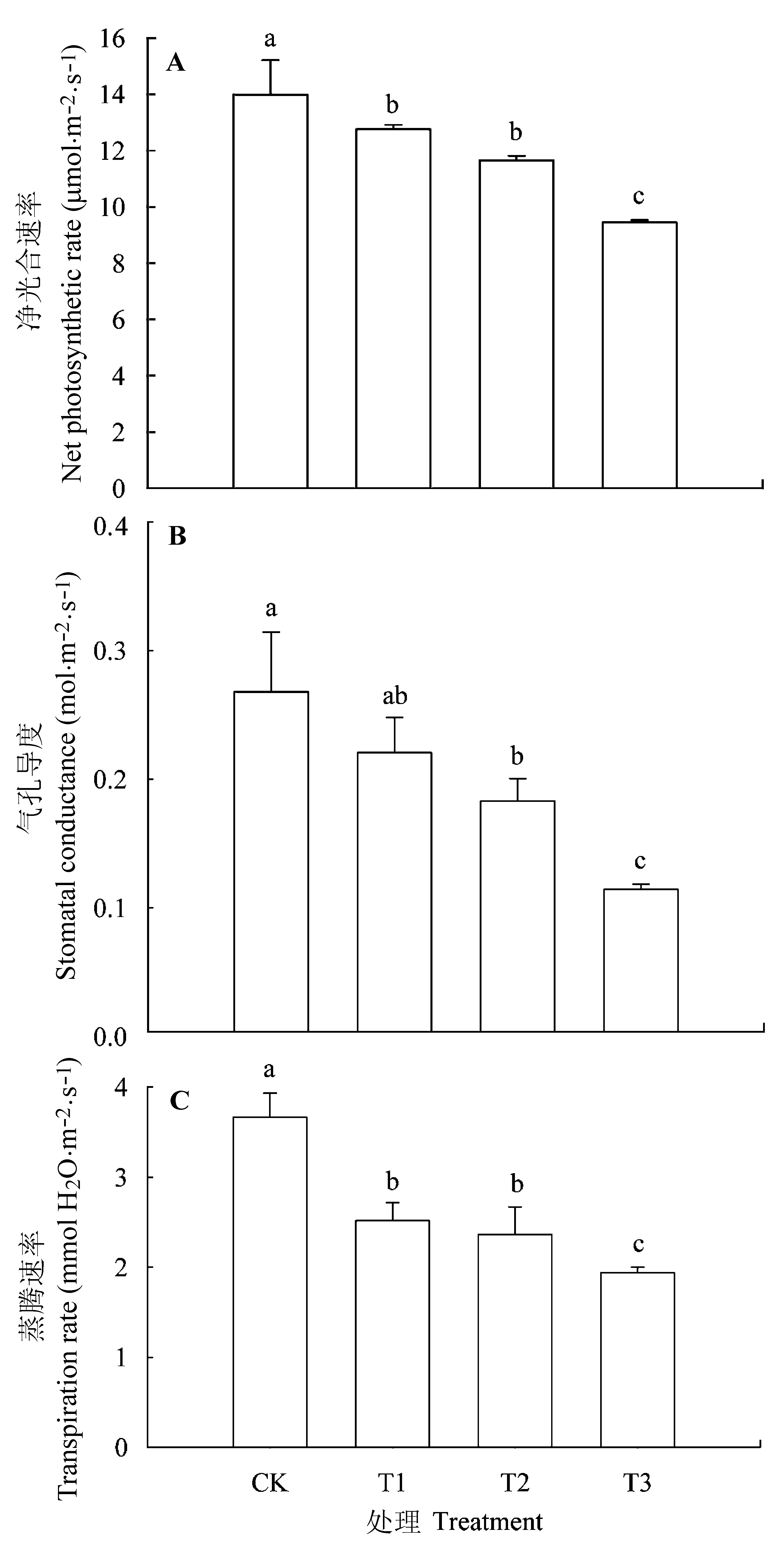
图7 核桃幼苗在不同处理条件下气体交换的变异(平均值±标准误差)。CK, 正常供磷素+ pH 6.0; T1, 正常供磷素+ pH 3.0; T2, 不添加磷素+ pH 6.0; T3, 不添加磷素+ pH 3.0。 不同小写字母表示不同处理间差异显著(p < 0.05)。
Fig. 7 Variations of gas exchanges in Juglans regia seedlings under different treatments (mean ± SE). CK, normal phosphorus and pH 6.0; T1, normal phosphorus and pH 3.0; T2, phosphorous deficiency and pH 6.0; T3, phosphorus deficiency and pH 3.0. Different small letters indicate significant differences among treatments (p < 0.05).
| 处理 Treatment | CK | T1 | T2 | T3 |
|---|---|---|---|---|
| 导管密度 Vessel density (No.·mm-2) | 318 ± 41.35a | 236 ± 23.30b | 229 ± 20.88b | 175 ± 16.41c |
| 导管直径 Vessel diameter (μm) | 28.43 ± 3.32a | 27.74 ± 2.04a | 27.0 ± 2.31ab | 24.8 ± 1.84b |
表6 核桃幼苗叶柄木质部解剖结构(平均值±标准误差)
Table 6 Anatomical structure of petiole xylem in Juglans regia seedlings under different treatments (mean ± SE)
| 处理 Treatment | CK | T1 | T2 | T3 |
|---|---|---|---|---|
| 导管密度 Vessel density (No.·mm-2) | 318 ± 41.35a | 236 ± 23.30b | 229 ± 20.88b | 175 ± 16.41c |
| 导管直径 Vessel diameter (μm) | 28.43 ± 3.32a | 27.74 ± 2.04a | 27.0 ± 2.31ab | 24.8 ± 1.84b |
| Pn | Gs | Tr | Fv/Fm | ΦPSII | qP | NPQ | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | |||||||
| Phosphorus (P) | 48.676 | 0.000 | 19.368 | 0.000 | 20.750 | 0.000 | 15.241 | 0.001 | 20.073 | 0.000 | 26.746 | 0.000 | 22.131 | 0.000 | ||||||
| pH | 17.963 | 0.000 | 7.118 | 0.016 | 29.513 | 0.000 | 7.402 | 0.013 | 11.444 | 0.003 | 7.512 | 0.013 | 17.880 | 0.001 | ||||||
| P × pH | 1.447 | 0.245 | 0.247 | 0.626 | 4.459 | 0.052 | 0.013 | 0.910 | 0.066 | 0.800 | 0.748 | 0.398 | 0.184 | 0.674 | ||||||
表7 磷元素与pH值对核桃幼苗光合参数影响的双因素分析
Table 7 Two-way ANOVA for testing the effects of phosphorus and pH value on phosphorus characteristics in Juglans regia seedlings
| Pn | Gs | Tr | Fv/Fm | ΦPSII | qP | NPQ | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | F | Sig. | |||||||
| Phosphorus (P) | 48.676 | 0.000 | 19.368 | 0.000 | 20.750 | 0.000 | 15.241 | 0.001 | 20.073 | 0.000 | 26.746 | 0.000 | 22.131 | 0.000 | ||||||
| pH | 17.963 | 0.000 | 7.118 | 0.016 | 29.513 | 0.000 | 7.402 | 0.013 | 11.444 | 0.003 | 7.512 | 0.013 | 17.880 | 0.001 | ||||||
| P × pH | 1.447 | 0.245 | 0.247 | 0.626 | 4.459 | 0.052 | 0.013 | 0.910 | 0.066 | 0.800 | 0.748 | 0.398 | 0.184 | 0.674 | ||||||
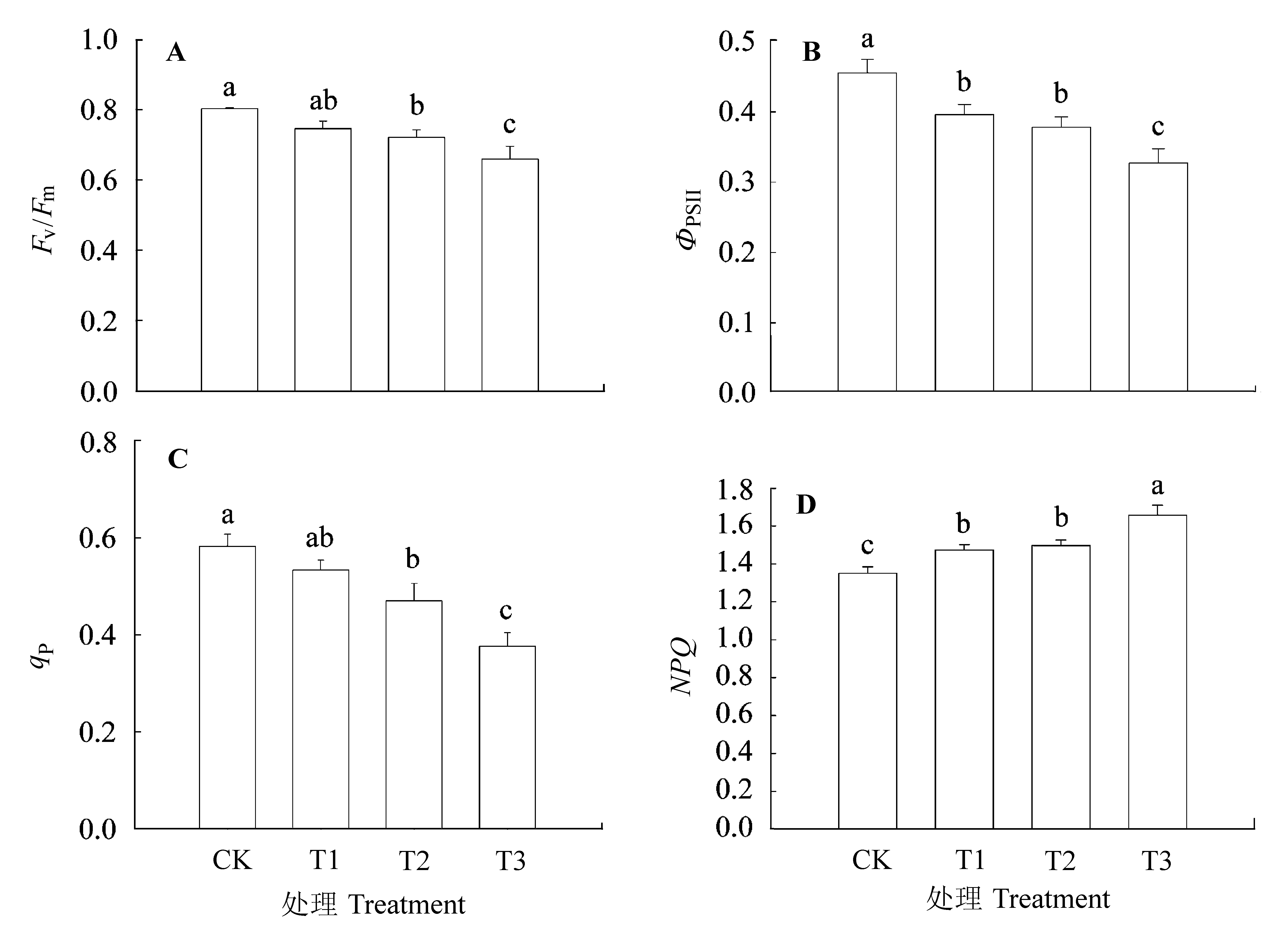
图8 不同处理条件下核桃幼苗叶绿素荧光参数(平均值±标准误差)。CK, 正常供磷素+ pH 6.0; T1, 正常供磷素+ pH 3.0; T2, 不添加磷素+ pH 6.0; T3, 不添加磷素+ pH 3.0。ΦPSII, PSII有效光化学量子效率; Fv/Fm, PSII最大光化学量子效率; NPQ, 非光化学淬灭; qP, 光化学淬灭。 不同小写字母表示不同处理间差异显著(p < 0.05)。
Fig. 8 Parameters of chlorophyll fluorescence in Juglans regia seedlings under different treatments (mean ± SE). CK, normal phosphorus and pH 6.0; T1, normal phosphorus and pH 3.0; T2, phosphorus deficiency and pH 6.0; T3, phosphorus deficiency and pH 3.0. ΦPSII, quantum yield of PSII; Fv/Fm, maximum efficiency of PSII; NPQ, non-photochemical quenching; qP, photochemical quenching. Different small letters indicate significant differences among treatments (p < 0.05).
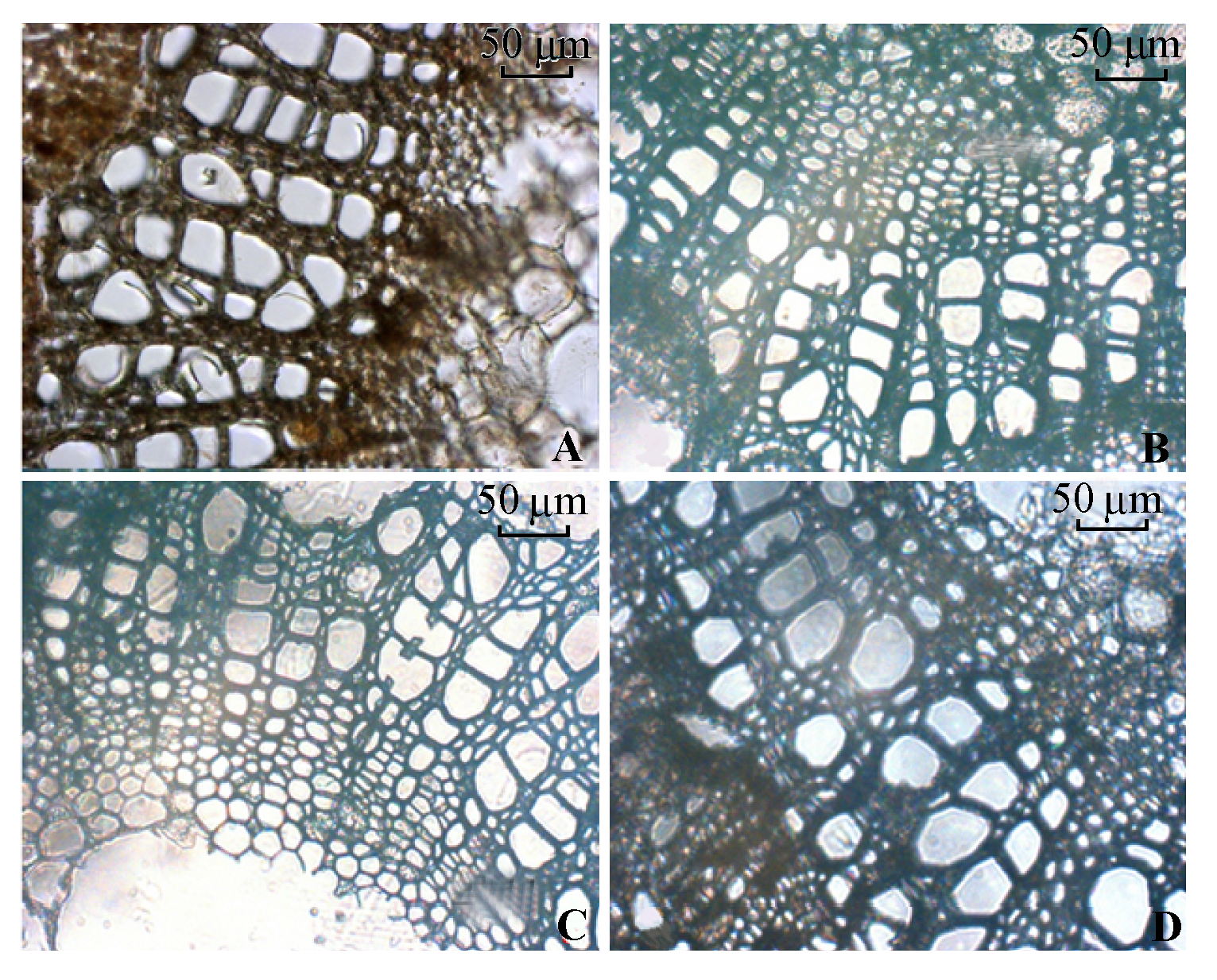
图9 不同处理条件下叶柄木质部导管的显微结构。 A, 正常供磷素+ pH 6.0。B, 正常供磷素+ pH 3.0。C, 不添加磷素 + pH 6.0。D, 不添加磷素+ pH 3.0。
Fig. 9 Microstructure of petiole xylem vessel under different treatments. A, Normal phosphorus and pH 6.0. B, Normal phosphorus and pH 3.0. C, Phosphorus deficiency and pH 6.0. D, Phosphorus deficiency and pH 3.0.
| 1 |
Brodribb TJ, Hill RS (2000). Increases in water potential gradient reduce xylem conductivity in whole plants. Evidence from a low pressure conductivity method. Plant Physiology, 123, 1021-1027.
URL PMID |
| 2 | Bucci SJ, Scholz FG, Goldstein G, Meinzer FC, Franco AC, Campanello PI, Villalobos-Vega R, Bustamante M, Miralles- Wilhelm F (2006). Nutrient availability constrains the hydraulic architecture and water relations of savannah trees. Plant, Cell & Environment, 29, 2153-2167. |
| 3 | Carvajal M, Cooke DT, Clarkson DT (1996). Responses of wheat plants to nutrient deprivation may involve the regulation of water-channel function. Planta, 199, 372-381. |
| 4 | Fan WG, Wang LX (2012). Photosynthetic response to different phosphorus levels on young Newhall navel orange trees. Journal of Fruit Science, 29, 166-170.(in Chinese with English abstract) |
| [ 樊卫国, 王立新 (2012). 纽荷尔脐橙幼树对不同供磷水平的光合响应. 果树学报, 29, 166-170.] | |
| 5 |
Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD (2004). Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biology, 6, 269-279.
DOI URL PMID |
| 6 | Gallé A, Haldimann P, Feller U (2007). Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytologist, 174, 799-810. |
| 7 |
Gulías J, Flexas J, Abadía A, Madrano H (2002). Photosynthetic responses to water deficit in six Mediterranean sclerophyll species: possible factors explaining the declining distribution of Rhamnus ludovici-salvatoris an endemic Balearic species. Tree Physiology, 22, 687-697.
DOI URL PMID |
| 8 |
Gunsé B, Poschenrieder C, Barcelo J (1997). Water transport properties of roots and root cortical cells in proton- and Al-stressed maize varieties. Plant Physiology, 113, 595-602.
DOI URL PMID |
| 9 |
Guo SL, Yan XF, Bai B, Yu S (2005). Responses of larch seedling’s photosynthetic characteristics to nitrogen and phosphorus deficiency. Chinese Journal of Applied Ecology, 16, 589-594.(in Chinese with English abstract)
URL PMID |
|
[ 郭盛磊, 阎秀峰, 白冰, 于爽 (2005). 落叶松幼苗光合特性对氮和磷缺乏的响应. 应用生态学报, 16, 589-594.]
PMID |
|
| 10 | Hargrave KR, Kolb KJ, Ewers FW, Davis SD (1994). Conduit diameter and drought-induced embolism in Salvia mellifera Greene (Labiatae). New Phytologist, 126, 695-705. |
| 11 |
Harvey HP, van den Driessche R (1997). Nutrition, xylem cavitation and drought resistance in hybrid poplar. Tree Physiology, 17, 647-654.
DOI URL PMID |
| 12 |
Horswill P, Sullivan O, PhoenixGK, Lee JA, Leake JR (2008). Base cation depletion, eutrophication and acidification of species-rich grasslands in response to long-term simulated nitrogen deposition. Environmental Pollution, 155, 336-349.
DOI URL PMID |
| 13 |
Kamaluddin M, Zwiazek JJ (2004). Effects of root medium pH on water transport in paper birch (Betula papyrifera) seedlings in relation to root temperature and abscisic acid treatments. Tree Physiology, 24, 1173-1180.
DOI URL PMID |
| 14 |
Kang SZ, Zhang JH (2004). Controlled alternate partialrootzone irrigation: its physiological consequences andimpact on water use efficiency. Journal of Experimental Botany, 55, 2437-2446.
DOI URL PMID |
| 15 | Lowther JR (1980). Use of a single sulphuric acid-hydrogen peroxide digest for the analysis of Pinus radiata needles. Communications in Soil Science & Plant Analysis, 11, 175-188. |
| 16 | Lu RK, Shi ZY, Qian CL (2000). Decline of phosphorus availability with time in soils. Acta Pedologica Sinica, 37, 323-329.(in Chinese with English abstract) |
| [ 鲁如坤, 时正元, 钱承梁 (2000). 磷在土壤中有效性的衰减. 土壤学报, 37, 323-329.] | |
| 17 |
Lu YM, Equiza MA, Deng X, Tyree MT (2010). Recovery of Populus tremuloides seedlings following severe drought causing total leaf mortality and extreme stem embolism. Physiologia Plantarum, 140, 246-257.
URL PMID |
| 18 |
Mai BR, Zheng YF, Liang J, Liu X, Li L, Zhong YC (2008). Effects of simulated acid rain on leaf photosynthate, growth, and yield of wheat. Chinese Journal of Applied Ecology, 19, 2227-2233.(in Chinese with English abstract)
URL PMID |
|
[ 麦博儒, 郑有飞, 梁骏, 刘霞, 李璐, 钟燕川 (2008). 模拟酸雨对小麦叶片同化物, 生长和产量的影响. 应用生态学报, 19, 2227-2233.]
PMID |
|
| 19 | Mao DR ( 1994). Research Methods of Plant Nutrition. Beijing Agricultural University Press, Beijing. |
| [ 毛达如(1994). 植物营养研究方法. 北京农业大学出版社, 北京.] | |
| 20 |
Maxwell K, Johnson GN (2000). Chlorophyll fluorescence―a practical guide. Journal of Experimental Botany, 51, 659-668.
URL PMID |
| 21 |
Mukherjee SK, Asanuma S (1998). Possible role of cellular phosphate pool and subsequent accumulation of inorganic phosphate on the aluminum tolerance in Bradyrhizobium japonicum. Soil Biology & Biochemistry, 30, 1511-1516.
DOI URL |
| 22 |
Ögren E (1990). Evaluation of chlorophyll fluorescence for drought stress in willow leaves. Plant Physiology, 93, 1280-1285.
DOI URL PMID |
| 23 |
Plavcová L, Hacke UG (2012). Phenotypic and developmental plasticity of xylem in hybrid poplar saplings subjected to experimental drought, nitrogen fertilization, and shading. Journal of Experimental Botany, 63, 6481-6491.
DOI URL PMID |
| 24 | Qiu DL, Liu XH (2000). Effect of simulated acid rain on the chlorophyll a fluorescence characteristic of longan (Dimorcarpus longana) leaves. Acta Horticulturae Sinica, 27, 177-181.(in Chinese with English abstract) |
| [ 邱栋梁, 刘星辉 (2000). 模拟酸雨对龙眼叶片叶绿素a荧光特性的影响. 园艺学报, 27, 177-181.] | |
| 25 | Sant’Anna-Santos BF, da Silva LC, Azevedo AA, de Araújo JM, Alves EF, da Silva EAM, Aguiara R (2006). Effects of simulated acid rain on the foliar micromorphology and anatomy of tree tropical species. Environmental and Experimental Botany, 58, 158-168. |
| 26 |
Santiago LS, Goldstein G, Meinzer FC, Fisher JB, Machado K, Woodruff D, Jones T (2004). Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia, 140, 543-550.
DOI URL PMID |
| 27 | Sperry JS, Donnelly JR, Tyree MT (1988). A method for measuring hydraulic conductivity and embolism in xylem. Plant, Cell & Environment, 11, 35-40. |
| 28 | Sperry JS, Hacke UG, Oren R, Comstock JP (2002). Water deficits and hydraulic limits to leaf water supply. Plant, Cell & Environment, 25, 251-263. |
| 29 | Steudle E (2000). Water uptake by plant roots: an integration of views. Plant and Soil, 226, 45-56. |
| 30 |
Szabó I, Bergantino E, Giacometti GM (2005). Light and oxygenic photosynthesis: energy dissipation as a protection mechanism against photo-oxidation. EMBO Reports, 6, 629-634.
DOI URL PMID |
| 31 |
Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu DT, Bligny R, Maurel C (2003). Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature, 425, 393-397.
DOI URL PMID |
| 32 | Tyree MT, Zimmermann MH (2000). Xylem Structure and the Ascent of Sap. Springer, Berlin. |
| 33 | Vance CP, Uhde C, Allan DL (2003). Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist, 157, 423-447. |
| 34 |
Vander W, Sherwin CH, Pammenter NW (2000). Xylem hydraulic characteristics of subtropical trees from contrasting habitats grown under identical environmental conditions. New Phytologist, 145, 51-59.
DOI URL |
| 35 |
Wan X, Zwiazek JJ (1999). Mercuric chloride effects on root water transport in aspen seedlings. Plant Physiology, 121, 939-946.
DOI URL PMID |
| 36 | Wang L, Feng JX, Wang SX, Jia CR, Wan XC (2013). The interaction of drought and slope aspect on growth of Quercus variabilis and Platycladus orientalis. Acta Ecologica Sinica, 33, 2425-2433.(in Chinese with English abstract) |
| [ 王林, 冯锦霞, 王双霞, 贾长荣, 万贤崇 (2013). 干旱和坡向的互作对栓皮栎和侧柏生长的影响. 生态学报, 33, 2425-2433.] | |
| 37 | Yang JF, He LY, Zuo XD, Liu YF, Wu ZH, Zhang AQ, Zhao HE, Liu W, Yan C, Men YY (2009). Phosphorous nutritional characteristics of rice in P-deficient soils with different pH values. Plant Nutrition and Fertilizer Science, 15, 62-68.(in Chinese with English abstract) |
| [ 杨建峰, 贺立源, 左雪冬, 刘艳飞, 吴照辉, 章爱群, 赵会娥, 刘伟, 严昶, 门玉英 (2009). 不同pH低磷土壤上水稻磷营养特性研究. 植物营养与肥料学报, 15, 62-68.] | |
| 38 | Yuan J (2013). Study on Adaptive Mechanism of Camellia oleifera to Low-phosphorus Environment. PhD dissertation, Beijing Forestry University, Beijing. |
| [ 袁军(2013). 油茶低磷适应机理研究. 博士学位论文, 北京林业大学, 北京.] | |
| 39 | Zhang H, Yang YK, Xie DT, Wang DY (2007). Effect of acid rain on leaching loss of nitrogen and phosphorus. Journal of Soil and Water Conservation, 21, 22-25.(in Chinese with English abstract) |
| [ 张华, 杨永奎, 谢德体, 王定勇 (2007). 酸雨对紫色土氮磷淋失的影响. 水土保持学报, 21, 22-25.] | |
| 40 | Zhang SR, Gao RF (2000). Ecophysiological characteristics of photosynthesis of hybrid poplar clones under light stress. Acta Phytoecologica Sinica, 28, 528-533. |
| [ 张守仁, 高荣孚 (2004). 光胁迫下杂种杨无性系光合生理生态特性的研究. 植物生态学报, 24, 528-533.] | |
| 41 | Zhang SR, Gao RF, Wang LJ (2004). Response of oxygen evolution activity of photosystem II, photosynthetic pigments and chloroplast ultrastructure of hybrid poplar clones to light stress. Acta Phytoecologica Sinica, 28, 143-149. |
| [ 张守仁, 高荣孚, 王连军 (2004). 杂种杨无性系的光系统Ⅱ放氧活性、光合色素及叶绿体超微结构对光胁迫的响应. 植物生态学报, 28, 143-149.] |
| [1] | 许泽海 赵燕东. 生长季五角枫茎干水分含量序列特征及其影响因素解译[J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [2] | 周建 王焓. 森林径级结构研究:从统计描述到理论演绎[J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [3] | 范宏坤, 曾涛, 金光泽, 刘志理. 小兴安岭不同生长型阔叶植物叶性状变异及权衡[J]. 植物生态学报, 2024, 48(3): 364-376. |
| [4] | 施梦娇, 李斌, 伊力塔, 刘美华. 美洲黑杨幼苗生长和生理生态指标对干旱-复水响应的性别差异[J]. 植物生态学报, 2023, 47(8): 1159-1170. |
| [5] | 吴晨, 陈心怡, 刘源豪, 黄锦学, 熊德成. 增温对森林细根生长、死亡及周转特征影响的研究进展[J]. 植物生态学报, 2023, 47(8): 1043-1054. |
| [6] | 吴帆, 吴晨, 张宇辉, 余恒, 魏智华, 郑蔚, 刘小飞, 陈仕东, 杨智杰, 熊德成. 增温对成熟杉木人工林不同季节细根生长、形态及生理代谢特征的影响[J]. 植物生态学报, 2023, 47(6): 856-866. |
| [7] | 汪晶晶, 王嘉浩, 黄致云, Vanessa Chiamaka OKECHUKW, 胡蝶, 祁珊珊, 戴志聪, 杜道林. 不同氮水平下内生固氮菌对入侵植物南美蟛蜞菊生长策略的影响[J]. 植物生态学报, 2023, 47(2): 195-205. |
| [8] | 刘美君, 陈秋文, 吕金林, 李国庆, 杜盛. 黄土丘陵区辽东栎和刺槐树干径向生长与微变化季节动态特征[J]. 植物生态学报, 2023, 47(2): 227-237. |
| [9] | 安凡, 李宝银, 钟全林, 程栋梁, 徐朝斌, 邹宇星, 张雪, 邓兴宇, 林秋燕. 不同种源刨花楠苗木生长与主要功能性状对氮添加的响应[J]. 植物生态学报, 2023, 47(12): 1693-1707. |
| [10] | 刘艳杰, 刘玉龙, 王传宽, 王兴昌. 东北温带森林5个羽状复叶树种叶成本-效益关系比较[J]. 植物生态学报, 2023, 47(11): 1540-1550. |
| [11] | 张志山, 韩高玲, 霍建强, 黄日辉, 薛书文. 固沙灌木柠条锦鸡儿和中间锦鸡儿木质部导水与叶片光合能力对土壤水分的响应[J]. 植物生态学报, 2023, 47(10): 1422-1431. |
| [12] | 李变变, 张凤华, 赵亚光, 孙秉楠. 不同刈割程度对油莎豆非结构性碳水化合物代谢及生物量的影响[J]. 植物生态学报, 2023, 47(1): 101-113. |
| [13] | 朱明阳, 林琳, 佘雨龙, 肖城材, 赵通兴, 胡春相, 赵昌佑, 王文礼. 云南轿子山不同海拔急尖长苞冷杉径向生长动态及其低温阈值[J]. 植物生态学报, 2022, 46(9): 1038-1049. |
| [14] | 李一丁, 桑清田, 张灏, 刘龙昌, 潘庆民, 王宇, 刘伟, 袁文平. 内蒙古半干旱地区空气和土壤加湿对幼龄樟子松生长的影响[J]. 植物生态学报, 2022, 46(9): 1077-1085. |
| [15] | 魏瑶, 马志远, 周佳颖, 张振华. 模拟增温改变青藏高原植物繁殖物候及植株高度[J]. 植物生态学报, 2022, 46(9): 995-1004. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19